‘Frontal AD’ a Misnomer? Behavioral Variant Has Minimal Frontal Atrophy
Quick Links
As if grappling with memory loss isn’t hard enough, a subset of people with Alzheimer’s disease have behavioral problems that rival their cognitive ones. Previously dubbed “frontal variant AD,” this rare form of the disease actually spares the frontal lobes and instead wreaks havoc in temporoparietal regions, according to a study published July 2 in Brain. The paper reports a retrospective analysis of the largest sample to date of pathological or biomarker-confirmed Alzheimer’s patients with a behavioral or executive presentation. It revealed that these people do share these non-memory symptoms with frontotemporal dementia (FTD) patients; however, unlike FTD patients, sufferers of these forms of AD also have severe memory impairments and plaque/tangle pathology. The cause of these patients’ behavioral symptoms is still unclear, but for now the researchers propose scrapping the “frontal AD” moniker and replacing it with one that describes the symptoms, rather than the supposed location of the pathology.
Led by Gil Rabinovici and William Seeley at the University of California, San Francisco, along with Philip Scheltens and Yolande Pijnenburg at VU University Medical Center in Amsterdam, the researchers call these variants bAD (for behavioral variant of AD) and deAD (for dysexecutive variant of AD) and plan to further define their characteristics to enable more accurate diagnoses.
“Given the behavioral symptoms of these patients, we were surprised to find little frontal involvement,” said first author Rik Ossenkoppele, a postdoc who splits his time between UCSF and VUMC. “A description based on their clinical symptoms is much more accurate than one based on the anatomical location of atrophy.”
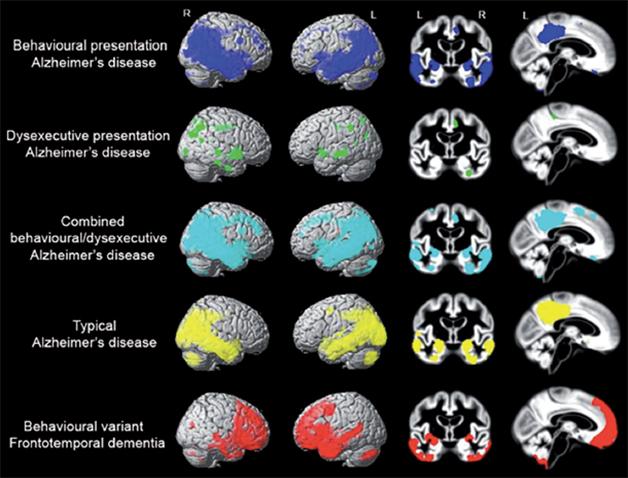
Not Full Frontal. Atrophy patterns in people with behavioral and/or dysexecutive variants of Alzheimer’s resemble those of typical AD. People with FTD have more frontal atrophy. (Image courtesy of the author and Oxford University Press. From the journal Brain, July 2015 [http://brain.oxfordjournals.org/content/early/2015/07/02/brain.awv191]).
As the command center of behavior and executive function, the frontal lobe regulates the way people respond to emotions and make decisions and plans. People with behavioral variant FTD (bvFTD) have profound neurodegeneration in the frontal and temporal lobes. Many show behavioral symptoms such as apathy, lack of empathy or appropriate inhibition, and obsessive/compulsive tendencies, but their memory initially remains intact.
In previous studies, amyloid PET or autopsy showed 10 to 40 percent of people diagnosed with bvFTD to have AD pathology instead (see Ossenkoppele et al., 2013 and Forman et al., 2006). On the flip side, researchers reported 16 years ago that some AD patients had behavioral or executive impairments similar to people with bvFTD (see Johnson et al., 1999). A smattering of studies since then has confirmed the existence of AD variants either marked by predominant executive impairments (such as difficulty solving problems) and/or behavioral symptoms, accompanied by some form of frontal pathology. Alas, there is no consensus on the patterns of AD pathology that underlie these disorders (see Dickerson et al., 2011, Balasa et al., 2011, Mendez et al., 2013, and Blennerhasset et al., 2014).
The combination of so-called frontal symptoms and some reports of frontal pathology led researchers to refer to the variant as “frontal AD.” It is described as such in the International Working Group’s diagnostic criteria (see Dubois et al., 2014).
Ossenkoppele and colleagues wanted to conduct a larger study that would cut through the confusion of previous small ones. They drew upon a large database of patient records at both UCSF and VUMC going back to 1999. They defined bAD as patients with amyloid PET or post-mortem neuropathological evidence of AD who had either been clinically diagnosed with bvFTD, given a mixed diagnosis of bvFTD and AD, or who were diagnosed with “frontal AD.” Fifty-five patients met those criteria. The researchers added 29 AD patients whose executive function was particularly impaired, and labeled them as having deAD. For comparison, they included 58 people with typical amnestic AD, 59 with pathologically confirmed bvFTD, and 61 cognitively normal age-matched controls. The researchers searched for patterns within neuropsychological, cognitive, genetic, and imaging data among these groups. Despite their abnormal behavior, just over half of bAD patients initially presented with cognitive symptoms such memory and executive problems. Half met international criteria for bvFTD, because they displayed at least three of six behavioral symptoms characteristic of that disorder. Apathy was most common, followed by disinhibition and loss of empathy. However, other FTD symptoms such as hyperorality and compulsion were less common in bAD patients. A 54 year-old man whom Ossenkoppele described as typical bAD came to the clinic after experiencing a 2-year decline in memory followed by personality changes, aggression toward family members, and a general lack of interest in everyday life. This patient only met 2 of 6 FTD criteria and had positive AD biomarkers, so was diagnosed with “frontal AD.”
In the case of deAD, more than 80 percent of patients started out having cognitive symptoms, such as reduced memory and executive function. Most of the deAD patients had only one or two behavioral symptoms associated with bvFTD, so were not misdiagnosed with that disorder. However, nine of the deAD patients displayed three or more bvFTD behavioral symptoms and therefore met the criteria for both bAD and deAD.
Both bAD and deAD patients had a similar level of memory impairment as people with typical AD, but more executive impairment. Compared to people with bvFTD, both bAD and deAD patients had worse executive function and memory scores. About 40 percent of bAD and 60 percent of deAD patients carried at least one ApoE4 allele, compared to more than 70 percent of typical AD patients, 19 percent of bvFTD patients, and 17 percent of controls.
The researchers next compared structural MRI scans between patients in each group versus controls (see image above.) As expected, people with typical AD had temporoparietal atrophy, in the posterior cingulate, precuneus, and medial temporal lobes. People with FTD, on the other hand, had atrophy in frontotemporal regions, including the anterior cingulate cortex, frontal insula, and anterior temporal lobes. Surprisingly, patients with bAD had a similar temporoparietal pattern of damage as those with typical AD. Some had a bit of frontal atrophy as well, however those atrophy patterns fell short of statistical thresholds. People with deAD were also similar to those with typical AD in their lack of frontal atrophy, with the exception of some shrinkage of the anterior cingulate cortex. People with typical AD had more deterioration in their occipital cortices than did bAD or deAD patients.
Autopsy data was available for 24 bAD and/or deAD patients. They had significant amyloid plaques and neurofibrillary tangles, the researchers found. The researchers did not compare the distribution patterns of AD pathology in the patients, but plan on doing so in future studies.
If bAD and deAD patients lack the frontal atrophy that causes behavioral symptoms in bvFTD patients, then what explains their behavioral symptoms? Perhaps the patients do have some form of frontal neurodegeneration or pathology that causes no obvious atrophy? This is unlikely, Ossenkoppele said, as regions of the brain that affect memory already had atrophy, and bvFTD patients displayed atrophy in frontal regions that correlate with behavioral and executive problems. Ossenkoppele and colleagues are starting to perform tau imaging on current patients to look for frontal pathology, and differences between patient groups.
Another possibility is that bAD/deAD patients have a less “frontal reserve,” meaning smaller amounts of pathology could interfere with neural function. The researchers did find that bAD and deAD patients had higher rates of previous traumatic brain injuries and depression than did typical AD patients. These past insults could have made their brain more vulnerable to small levels of frontal pathology or, in the case of depression, indicate pre-existing frontal vulnerability. Ossenkoppele said the researchers are combing more thoroughly for previous disorders or personality quirks in the patients via detailed questionnaires. “This is the best way to determine whether they already had some vulnerabilities in the frontal system,” he said.
Finally, people with bAD or deAD could have damaged circuitry connecting the frontal regions of the brain, rather than all-out neurodegeneration in those areas, Ossenkoppele speculated. “The field of neuroscience is shifting toward more network thinking, rather than focusing on a localized attribution of functions,” he said. He is planning to conduct functional MRI studies to pinpoint possible network disconnections in the patients. This work will need to rely heavily on past and present participants to conduct future studies, as bAD and/or deAD patients account for only 2 percent of AD patients.
“The careful clinicopathologic analyses in the report by Ossenkoppele and colleagues shed further light on the factors that underlie the intriguing syndromic diversity of neurodegenerative diseases,” commented Marek-Marsel Mesulam of Northwestern University in Chicago. The bAD/deAD variants join a list of other AD variants, including primary progressive aphasia (PPA), which affects language, and posterior cortical atrophy (PCA), which attacks visuospatial function (see Jul 2012 news and Jul 2013 news). PPA, a disorder Mesulam characterized, can be caused by any of eight different underlying pathologies, including AD. The current study illustrates the flip side of the coin, Mesulam wrote: “The same neuropathologic entity can give rise to multiple clinical syndromes.”—Jessica Shugart
References
News Citations
- Researchers Join to Draw Posterior Cortical Atrophy Out of Shadows
- Atrophy of Distinct Brain Networks May Explain Alzheimer’s Variants
Paper Citations
- Ossenkoppele R, Prins ND, Pijnenburg YA, Lemstra AW, van der Flier WM, Adriaanse SF, Windhorst AD, Handels RL, Wolfs CA, Aalten P, Verhey FR, Verbeek MM, van Buchem MA, Hoekstra OS, Lammertsma AA, Scheltens P, van Berckel BN. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement. 2012 Nov 16; PubMed.
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, Chatterjee A, Hurtig HI, Karlawish JH, Rosen HJ, Van Deerlin V, Lee VM, Miller BL, Trojanowski JQ, Grossman M. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006 Jun;59(6):952-62. PubMed.
- Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999 Oct;56(10):1233-9. PubMed.
- Dickerson BC, Wolk DA, . Dysexecutive versus amnesic phenotypes of very mild Alzheimer's disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry. 2011 Jan;82(1):45-51. PubMed.
- Balasa M, Gelpi E, Antonell A, Rey MJ, Sánchez-Valle R, Molinuevo JL, Lladó A, . Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology. 2011 May 17;76(20):1720-5. PubMed.
- Mendez MF, Joshi A, Tassniyom K, Teng E, Shapira JS. Clinicopathologic differences among patients with behavioral variant frontotemporal dementia. Neurology. 2013 Feb 5;80(6):561-8. PubMed.
- Blennerhassett R, Lillo P, Halliday GM, Hodges JR, Kril JJ. Distribution of Pathology in Frontal Variant Alzheimer's Disease. J Alzheimers Dis. 2013 Oct 10; PubMed.
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014 Jun;13(6):614-29. PubMed.
External Citations
Further Reading
Primary Papers
- Ossenkoppele R, Pijnenburg YA, Perry DC, Cohn-Sheehy BI, Scheltens NM, Vogel JW, Kramer JH, van der Vlies AE, Joie RL, Rosen HJ, van der Flier WM, Grinberg LT, Rozemuller AJ, Huang EJ, van Berckel BN, Miller BL, Barkhof F, Jagust WJ, Scheltens P, Seeley WW, Rabinovici GD. The behavioural/dysexecutive variant of Alzheimer's disease: clinical, neuroimaging and pathological features. Brain. 2015 Sep;138(Pt 9):2732-49. Epub 2015 Jul 2 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Sorbonne University - APHP - Pitié-Salpêtrière Hospital
In the new framework for the diagnosis of Alzheimer’s disease (AD) that the International Working Group has proposed since 2007, we introduced the concept that AD is a “clinical-biological entity”. This means that AD should be identified in patients first on the basis of a specific clinical phenotype. The most frequent phenotype is the “amnestic syndrome of the hippocampal type” (that we have described and defined as “typical AD”).
Other additional clinical presentations can be encountered: they were described in 2010 as atypical forms of AD, which include the posterior variant of AD, the logopenic variant of AD and the frontal variant of AD (Dubois et al., 2010). These clinical phenotypes are related to AD on the positivity of biomarker of AD pathology (specific changes in the CSF or positive amyloid PET). In our mind, the role of biomarkers is mainly to confirm the clinical evidence for AD.…More
Therefore, it is mostly important to better describe the clinical phenotypes of AD. This is the aim of this paper and this is, no doubt, a significant contribution to the field.
Northwestern University
This timely paper illustrates two important principles relevant to dementia research in general. The first is that the same neuropathologic entity can give rise to multiple clinical syndromes, some more typical than others. The second is that Alzheimer’s disease has subtypes. They include not only the typical amnestic variant but also variants that predominantly undermine language (as in the primary progressive aphasia [PPA] syndrome), visuospatial function (as in the posterior cortical atrophy syndrome), and, as shown in this paper, executive/behavioral domains. These principles were illustrated by PPA, which can be caused by at least eight different pathological entities, including Alzheimer’s. Furthermore, the Alzheimer pathology that causes PPA displays an atypical asymmetry of neurofibrillary tangles, is not linked to ApoE4 as a risk factor, and does not display the relatively high frequency of TDP-43 precipitates detected in typical forms of AD (Bigio et al., 2010; Gefen et al., 2012; Mesulam et al., 2014). The careful clinicopathologic analyses in the report by Ossenkoppele and colleagues shed further light on the factors that underlie the intriguing syndromic diversity of neurodegenerative diseases.…More
References:
Bigio EH, Mishra M, Hatanpaa KJ, White CL, Johnson N, Rademaker A, Weitner BB, Deng HX, Dubner SD, Weintraub S, Mesulam M. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol. 2010 Jul;120(1):43-54. PubMed.
Gefen T, Gasho K, Rademaker A, Lalehzari M, Weintraub S, Rogalski E, Wieneke C, Bigio E, Geula C, Mesulam MM. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain. 2012 May;135(Pt 5):1554-65. PubMed.
Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain. 2014 Apr;137(Pt 4):1176-92. Epub 2014 Feb 25 PubMed.
Make a Comment
To make a comment you must login or register.