First Plasma Assay for Oligomeric Aβ Binds Synaptotoxic Species
Quick Links
Scientists agree that Aβ oligomers damage synapses, but their exact role in Alzheimer’s disease has been maddeningly hard to pin down because they are extremely difficult to detect. In the September 22 Alzheimer’s & Dementia, researchers led by Dennis Selkoe at Brigham and Women’s Hospital, Boston, debuted a new assay they hope will solve this problem. In collaboration with Trebor Lawton at biotech start-up Abyssinia Biologics in Durham, New Hampshire, Selkoe developed an oligomeric Aβ sandwich immunoassay that recognizes small soluble species found in body fluids.
- New immunoassay binds small soluble Aβ oligomers with high affinity.
- These oligomers are toxic to synapses.
- This ELISA is the first test sensitive enough to detect oligomeric Aβ in plasma.
With a sensitivity 100 times greater than a previous version, this new assay for the first time allows researchers to measure Aβ oligomers not only in cerebrospinal fluid but in plasma, too. Because blood samples are easier to obtain than CSF, the assay could open the door to routine measurements of oligomeric Aβ. “We now have a plasma assay for what we think is the bioactive, synaptotoxic form of Aβ,” Selkoe told Alzforum.
“This assay will be an important tool to explore the pathophysiological role of oligomeric Aβ directly in AD patients, and in longitudinal studies on unimpaired elderly at risk for AD,” Kaj Blennow at the University of Gothenburg, Sweden, wrote to Alzforum (full comment below).

Antibody Sandwich. Antibody 71A1 is bound to magnetic beads (blue) and used to capture oligomeric Aβ (pink), which is then detected by a different antibody (3D6) labelled with a fluorophore (Alexa 647). [Courtesy of Liu et al., Alzheimer’s & Dementia.]
Selkoe and colleagues had previously developed an immunoassay that used the Aβ oligomer-specific antibody 1C22 (Yang et al., 2015; Jul 2018 news). It recorded a drop in oligomeric Aβ in the CSF of people taking the anti-amyloid antibody crenezumab (Jul 2018 news; Yang et al., 2019). Still, the low concentrations present in CSF were at the limit of quantification for this assay, and plasma concentrations were undetectable.
To make a more sensitive assay, Lawton generated new antibodies using residues 9-18 of Aβ as the antigen. He cyclized this fragment to mimic the likely three-dimensional structure of Aβ dimers. The new antibodies, 71A1 and 1G5, were 100-fold more sensitive for oligomers than monomers. When incorporated into a sandwich ELISA and tested on a synthetic Aβ preparation of Aβ-derived diffusible ligands, aka ADDLs, the new antibodies had similar sensitivity as 1C22, with a lower limit of detection of 0.6 pg/ml.
However, on biological material, 71A1 and 1G5 acted quite differently than 1C22. First author Lei Liu found that 1C22 immunoprecipitated far more Aβ from AD brain extract than did the new antibodies, but in CSF, the situation was reversed, with the new antibodies pulling down more material. Size chromatography revealed that oligomers in the CSF were much smaller than those found in brain. This implies that the new antibodies are selective for smaller species, while 1C22 recognizes larger forms. 71A1 had slightly higher affinity for oligomeric Aβ than did 1G5, and was used in subsequent experiments.
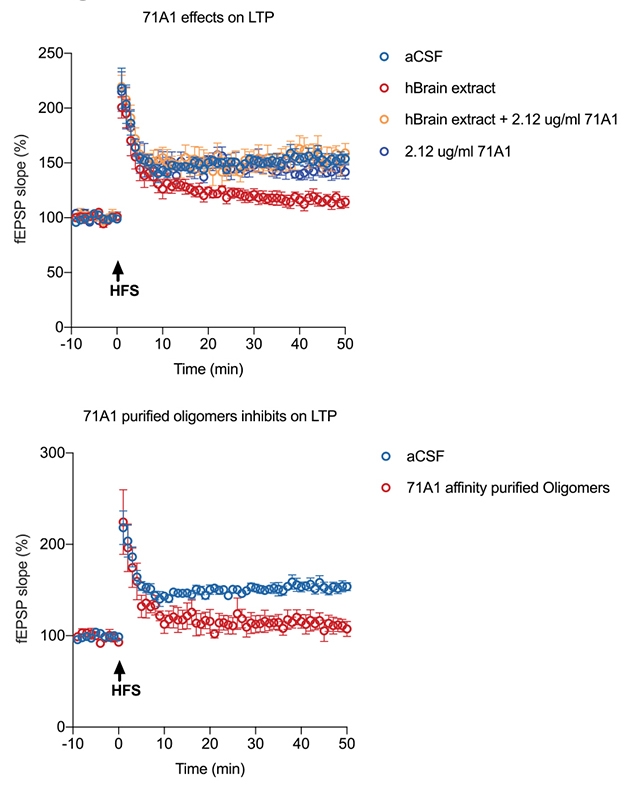
Capturing Synaptotoxicity. In mouse hippocampal slices, long-term potentiation of neural circuits (blue) is depressed by AD brain extract (red, top). However, incubating brain extract with antibody 71A1 abolishes its synaptotoxicity (yellow, top). Meanwhile, the material immunoprecipitated by 71A1 (red, bottom) is as synaptotoxic as the full brain extract. [Courtesy of Liu et al., Alzheimer’s & Dementia.]
The smaller Aβ species seems to be the synaptotoxic form. The small amount of material that 71A1 immunoprecipitated from AD brain was as damaging to synapses as the full brain extract. Conversely, pre-incubating brain extract with 71A1 neutralized it (see image above).
The authors tested the 71A1 ELISA on CSF samples from 36 people seen at the Brigham and Women’s memory disorders clinic. The assay produced a strong signal even when CSF samples were diluted, an important feature for developing a routine clinical test. Intriguingly, oligomeric Aβ levels in CSF correlated with total tau and p-tau181, suggesting a connection between Aβ toxicity and neurodegeneration.
What about plasma? In blood samples from 73 cognitively healthy participants in the Mayo Clinic Study of Aging, the 71A1 ELISA detected an average concentration of 43 pg/ml oligomeric Aβ, about 100-fold less than the average concentration in the BWH CSF samples. These numbers are based on using ADDLs to standardize the ELISA. Repeated measures on the same sample varied less than 20 percent.
In future work, the authors will test whether the assay can distinguish AD patients from healthy controls, and amyloid-positive from -negative participants, using samples from the Harvard Aging Brain Study. They will also test samples from the A4 trial to correlate oligomeric Aβ with other AD biomarkers such as amyloid PET, and to look for a potential effect of treatment on the biomarker.
Abyssinia Biologics plans to license the technology to a company that will commercialize the assay. Liu believes 71A1 also has potential as an immunotherapy, and is testing this idea on APPNLGF knock-in mice.—Madolyn Bowman Rogers
References
News Citations
- A Minority of Human Aβ Species are Toxic, Good Drug Targets
- On Target: Crenezumab Reduces Aβ Oligomers in CSF
Therapeutics Citations
Research Models Citations
Paper Citations
- Yang T, O'Malley TT, Kanmert D, Jerecic J, Zieske LR, Zetterberg H, Hyman BT, Walsh DM, Selkoe DJ. A highly sensitive novel immunoassay specifically detects low levels of soluble Aβ oligomers in human cerebrospinal fluid. Alzheimers Res Ther. 2015;7(1):14. Epub 2015 Mar 22 PubMed.
- Yang T, Dang Y, Ostaszewski B, Mengel D, Steffen V, Rabe C, Bittner T, Walsh DM, Selkoe DJ. Target engagement in an alzheimer trial: Crenezumab lowers amyloid β oligomers in cerebrospinal fluid. Ann Neurol. 2019 Aug;86(2):215-224. Epub 2019 Jun 22 PubMed.
Further Reading
News
- With Sudden Progress, Blood Aβ Rivals PET at Detecting Amyloid
- Is Shape of Aβ an Early Blood Biomarker for Alzheimer’s?
- After Plasma Aβ, Now Plasma P-Tau181 Shows Promise
- Elusive or Not, Aβ Oligomers Are in BioPharma Crosshairs
- Two Classes of Aβ Oligomers Act Differently in the Brain
- New Tack on Aβ Oligomer Role in Disease and Treatment
Primary Papers
- Liu L, Kwak H, Lawton TL, Jin SX, Meunier AL, Dang Y, Ostaszewski B, Pietras AC, Stern AM, Selkoe DJ. An ultra-sensitive immunoassay detects and quantifies soluble Aβ oligomers in human plasma. Alzheimers Dement. 2021 Sep 22; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Goteborg, Sahlgrenska University Hospital
Selkoe and co-workers present a new immunoassay for the quantification of Aβ oligomers in human CSF and plasma. After showing the specificity of two new oAβ-selective antibodies, they perform a thorough validation of this ultra-sensitive oAβ immunoassay, as well as the first results showing that it works to accurately quantify these key species of AD pathogenesis.
This assay will be an important tool to explore the pathophysiological role of oAβ directly in AD patients and in longitudinal studies on unimpaired elderly at risk for AD. Interesting outcomes may include how well plasma and CSF levels of oAβ correlate, and in which way plasma/CSF oAβ levels are associated with brain amyloidosis assessed by PET, specifically if a change in oAβ levels is evident before the PET signal reaches the threshold for positivity. Further, given the synaptotoxicity of oAβ shown in animal studies, data on how early in the AD continuum plasma/CSF oAβ levels change as compared to CSF levels of synaptic biomarkers would be exciting. In this respect, the finding in the present study of strong correlations between CSF oAβ levels and both CSF T-tau and P-tau181 is intriguing.
Nevertheless, an important piece has been added to the AD biomarker toolbox, giving promise on future important mechanistic insights on the early stages of AD pathophysiology.
Make a Comment
To make a comment you must login or register.