FDA Panel Rejects Neuronix Brain Stimulation Device
Quick Links
An advisory panel appointed by the FDA was nearly unanimous in recommending yesterday that Yokneam, Israel-based Neuronix not be allowed to market its neuroAD Therapy System in the U.S. Approved for AD in Europe, Australia, and Israel, the device combines repetitive transcranial magnetic stimulation (rTMS) and cognitive training in what it claims to be a memory-improving therapy for people in the early stages of Alzheimer’s disease. However, the company’s Phase 3 data of a six-week treatment trial missed its primary endpoint. As outlined in a brief FDA summary, “The Panel was in unanimous agreement that the probable benefits to health of the neuroAD Therapy System do not outweigh the probable risks to health.”
- Neuronix is seeking approval for its neuroAD TMS device.
- Its analysis of Phase 3 data was met with skepticism from an expert panel.
- The panel overwhelmingly suggested that the FDA not approve neuroAD for marketing in the U.S.
“There may be some value for TMS in cognitive therapy, but the current pivotal trial certainly didn’t demonstrate it,” said David Knopman, Mayo Clinic, Rochester Minnesota, a voting member of the panel. “The sponsor needs to do a properly designed, larger, longer trial.”
To kick off the nine-hour meeting that took place in Gaithersburg, Maryland, on March 21, the company presented its interpretation of the Phase 3 data detailed previously at the AD/PD conference in 2017 (Apr 2017 conference news). After six weeks, patients receiving neuroAD therapy performed no better on the ADAS-Cog than patients who received sham stimulation. Physicians also gave the treated and sham-treated groups similar scores on the clinical global impression of change (CGIC). However, the company focused on the slight benefit detected later. Six weeks after the treatment had ended, a small improvement emerged in the rTMS group on both tests, mostly in people who had started the study with mild AD, i.e., with an ADAS-Cog score at or below 30. Higher scores indicate worse cognition on this scale and healthy people usually score five or below. No follow-up studies were done.
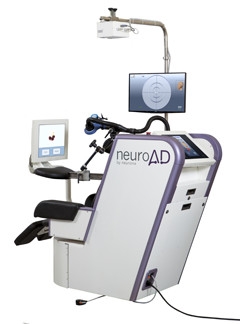
NeuroAD Therapy System. A magnetic coil delivers electrical current to certain brain regions and stimulates neurons to fire. A touchscreen delivers simultaneous cognitive training. [Courtesy of Neuronix.]
“They were making a lot out of some secondary and post hoc analyses that they had done and the panel just did not buy that,” said Kevin Duff, University of Utah, Salt Lake City, another voting member of the panel.
After the company’s pitch, the FDA presented a detailed reanalysis of data, one that followed more traditional rules of inference. For instance, it avoided multiple testing with planned or unplanned secondary analysis. “It was an excellent presentation,” Knopman said. It found little risk associated with the treatment, but saw no sign of efficacy.
A public comment period followed. European and Australian physicians reported seeing improvements in their patients over months and years. Family members of patients who had received the treatment talked about perceived benefits for their loved ones. One patient delivered a passionate plea for approval. However, other members of the public opposed approval in the U.S. based on the lack of demonstrated efficacy in placebo-controlled trials. The panel members agreed that the company failed to demonstrate clinical effectiveness, Knopman said. “We felt they didn’t meet that standard.”
“I think giving [this device] that FDA stamp of approval would give people the impression that the therapy really was effective,” said Duff. “People might cash in their 401K or mortgage their house to pay for something that we couldn’t really endorse as being effective.” The panel encouraged the sponsor to go back and do a study that focuses on people with milder disease, identified with the Mini-Mental State Examination (MMSE) instead of the ADAS-Cog. Members also suggested running a trial for six months to a year and including specific assessments for psychiatric adverse events.
“It was an intense meeting,” said Babak Tousi, Cleveland Clinic Lou Ruvo Center for Brain Health, one of the principal investigators of the Phase 3 trial. The news of the aducanumab failure broke the same morning and came after years of other failed trials (Mar 2019 news). “When we face all these failed trials, that’s the time to be more open and think out of the box,” said Tousi.
"We appreciate the thoughtful consideration of the neuroAD data as well as the needs of the Alzheimer's disease community of patients and caregivers,” said Eyal Baror, CEO of Neuronix, in a statement. “We plan to engage with the FDA to discuss a path forward.”—Gwyneth Dickey Zakaib
References
News Citations
- Transcranial Magnetic Stimulation for AD Boasts Success in Phase 3
- Biogen/Eisai Halt Phase 3 Aducanumab Trials
Therapeutics Citations
External Citations
Further Reading
Papers
- Andrade SM, de Oliveira EA, Alves NT, Dos Santos AC, de Mendonça CT, Sampaio DD, da Silva EE, da Fonsêca ÉK, de Almeida Rodrigues ET, de Lima GN, Carvalho J, da Silva JA, Toledo M, da Rosa MR, Gomes MQ, de Oliveira MM, Lemos MT, Lima NG, Inácio P, da Cruz Ribeiro E Rodrigues PM, Ferreira RG, Cavalcante R, de Brito Aranha RE, Neves R, da Costa E Souza RM, Portugal TM, Martins WK, Pontes V, de Paiva Fernandes TM, Contador I, Fernández-Calvo B. Neurostimulation Combined With Cognitive Intervention in Alzheimer's Disease (NeuroAD): Study Protocol of Double-Blind, Randomized, Factorial Clinical Trial. Front Aging Neurosci. 2018;10:334. Epub 2018 Nov 2 PubMed.
- Lin Y, Jiang WJ, Shan PY, Lu M, Wang T, Li RH, Zhang N, Ma L. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer's disease: A systematic review and meta-analysis. J Neurol Sci. 2019 Mar 15;398:184-191. Epub 2019 Jan 24 PubMed.
- Heath A, Taylor JL, McNerney MW. rTMS for the treatment of Alzheimer's disease: where should we be stimulating?. Expert Rev Neurother. 2018 Dec;18(12):903-905. Epub 2018 Oct 29 PubMed.
- Nguyen JP, Suarez A, Le Saout E, Meignier M, Nizard J, Lefaucheur JP. Combining cognitive training and multi-site rTMS to improve cognitive functions in Alzheimer's disease. Brain Stimul. 2018 May - Jun;11(3):651-652. Epub 2018 Feb 24 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.