Enter Aη: Alternative APP Cleavage Creates Synaptotoxic Peptide
Quick Links
Aβ42 has hogged the limelight in Alzheimer’s research, but growing evidence suggests that other fragments derived from amyloid precursor protein deserve close attention. Today in Nature, researchers led by Christian Haass at the German Center of Neurodegenerative Diseases, Munich, detail a new processing pathway for APP that generates a potent synaptotoxic fragment. The cleavage, which they call η (eta), cuts far N-terminal of the β-secretase site. It produces fragments about 92 or 108 amino acids long, which end at either the β- or α-secretase site, respectively. Although these fragments have been overlooked in previous studies, they are five to 10 times more abundant than Aβ in normal brains, the authors report. While the physiological role of these species is unknown, the longer of the Aη peptides, Aη-α, suppressed synaptic plasticity in vitro as well as neuronal activity in mouse brain. Moreover, levels of this synaptotoxic peptide rose in brain when the authors blocked BACE. “This could be problematic for BACE inhibitor therapy,” Haass suggested.
Commentators praised the rigor of the study and said the data make a convincing case for the existence of the η-secretase pathway. “This is a very careful and elegant study,” Dennis Selkoe at Brigham and Women’s Hospital, Boston, told Alzforum. At the same time, researchers said more work is needed to determine if these fragments could undercut the benefits of BACE inhibitor treatment, or if they contribute in some way to AD. “There are definitely more questions to answer before we can conclude whether this particular finding is important for Alzheimer’s,” said Gal Bitan at the University of California, Los Angeles.

New Kid on the Chopping Block.
The newly identified η-secretase cuts APP 92 amino acids before the β-secretase site. [Adapted from interactive diagram in Alzforum's mutation database.]
Researchers have known for some time that normal brains produce a menagerie of APP fragments. In the canonical pathway of Aβ production, BACE first cuts APP at the β-secretase site, located after position 671 of the longest APP isoform (see image at right). Then γ-secretase snips at variable sites within the transmembrane region to create fragments such as Aβ38, Aβ40, and Aβ42. In the non-amyloidogenic pathway, α-secretase clips APP after position 687 to generate harmless fragments. Additional peptides have surfaced in studies, including multiple short or truncated fragments of Aβ (see Apr 2010 news; Feb 2012 news; Mar 2013 conference news).
Other work has pointed to the existence of longer or extended forms of Aβ fragments. Selkoe and collaborator Dominic Walsh, also at Brigham and Women’s, identified N-terminally extended (NTE) Aβs that begin 40 amino acids before the β-secretase site and run to the γ-secretase site. These fragments appeared in media cultured by the Chinese hamster ovary cell line 7PA2, which expresses human V717F mutant APP and overproduces Aβ42. Like Aη, the NTE Aβ fragments impaired synaptic plasticity in hippocampal slices and their levels climbed in cell culture after BACE inhibition. The researchers have not determined how these are cleaved from APP at the N-terminal end, but because they end at the γ-secretase site, they represent distinct species from Aη.
Meanwhile, a group led by Erik Portelius, Kaj Blennow, and colleagues at the University of Gothenburg, Sweden, found yet a different class of extended APP fragments. The researchers reported more than 10 endogenous NTE species in human cerebrospinal fluid (CSF), the longest starting 63 amino acids before the β-secretase site, but all ending at the α-secretase site as Aη does. In the same 7PA2 cell line Selkoe and Walsh used, the Swedish group also showed that several of the NTE fragments ending at the α-secretase site increased after BACE inhibition (see May 2015 news).
For his part, Haass first saw evidence of the alternate Aη cleavage while working in Selkoe’s lab as a postdoc in the early 1990s. He noticed that endosomes and lysosomes of HEK293 cells accumulated a C-terminal fragment of APP with a mass of about 30 kilodaltons, much larger than the classic β-CTF and α-CTF cleavage fragments (see Haass et al., 1992). This suggested an N-terminal cleavage site upstream of the β site, but at the time, Haass did not pursue its origin. Recently, first author Michael Willem took up the search. Using antibodies to APP epitopes N-terminal to the Aβ sequence, Willem confirmed the existence of η-CTF, and of two Aη peptides in neuronal supernatants and 7PA2 media.
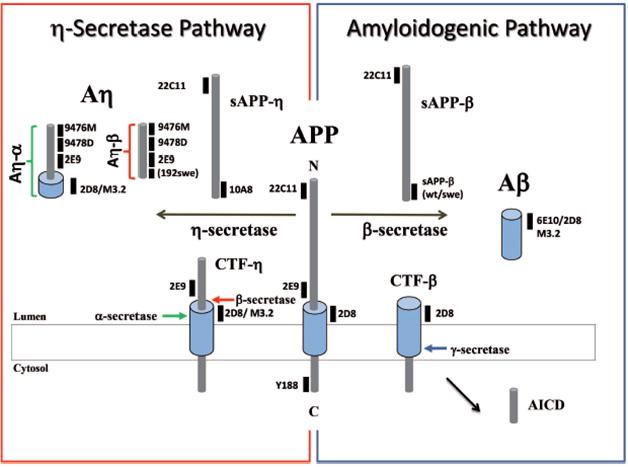
Peptide Profiles. The APP fragments generated by secretases and the antibodies that recognize them. [Courtesy of Willem et al., Nature.]
To nail down the identity of these peptides, the authors analyzed 7PA2 media by mass spectrometry. This revealed peptides that began at approximately position 580 of APP and ended at either the β or α site. These Aη fragments do not extend to the γ-secretase site, Haass emphasized. Therefore, they share little to no sequence in common with Aβ. The longer peptide, Aη-α, does include the first 16 amino acids of Aβ and also cross-reacts with some Aβ antibodies, such as 6E10, however.
Were these η fragments artifacts? Probably not, the scientists believe. Willem et al. identified η fragments in brain extracts from wild-type mice, suggesting they are physiological. Intriguingly, old mice contained less Aη than young mice. In collaboration with Rick Livesey at the University of Cambridge, England, Willem also found Aη species in neurons derived from human embryonic stem cells. The peptides also appear in CSF from adults. In the human neurons, Aη outnumbered Aβ tenfold, indicating Aη cleavage may be a major processing pathway. In CSF, Aη species were five times more abundant than Aβ.
Haass and colleagues next searched for the enzyme that produced Aη. Some matrix metalloproteinases (MMPs) are known to cleave APP at approximately the right region in vitro (see Higashi et al., 2003; Ahmad et al., 2006). One of these, membrane-type 5 MMP, is a membrane-bound metalloprotease found in neurons. Investigating it, the authors found that MT5-MMP knockout mice produced less Aη than wild-type mice do, as seen by western blot. The finding implies that MT5-MMP is an η-secretase, though other proteases clearly also contribute, Haass said.
A recent study from researchers led by Santiago Rivera at Aix-Marseille University, France, crossed MT5-MMP knockouts with 5xFAD mice. It reported less plaque burden and gliosis in the offspring, along with better memory. In cell cultures, MT5-MMP reportedly co-localized with APP and promoted β-secretase cleavage, in agreement with a role for the metalloprotease in APP processing (see Baranger et al., 2015).
Haass suggested that the relatively high abundance of Aη implies the fragments serve some physiological purpose, perhaps modulating neuronal function. To investigate this, the authors expressed Aη-α and Aη-β in a vector with a secretion signal in CHO cells, then added the conditioned media to hippocampal slice cultures. Neither peptide affected baseline synaptic transmission, but Aη-α suppressed long-term potentiation (LTP) as potently as Aβ dimers did. In fact, the original dimer preparations likely contained Aη, hence some of the LTP inhibition attributed to dimers may be due to Aη, the authors write.
What would Aη-α do in vivo? In collaboration with Arthur Konnerth at Technical University Munich, Germany, the authors imaged calcium currents in the neurons of live anesthetized animals, a newer measure of neuronal activity. They opened a 1 mm window in the skull over the hippocampus, infused the tissue with the peptide in a saline solution, then recorded calcium flux with two-photon imaging (see Busche et al., 2012). Aη-α quieted neuronal activity at levels as low as 5 nM. “This is so potent, we believe we’ve found something that has physiological action,” Willem told Alzforum. The finding was a surprise because Aβ, by contrast, hyperactivates neurons. The authors do not yet know why the two types of peptide affect LTP similarly, yet have opposite effects on calcium signaling in vivo. Willem found that Aη-β did not alter neural activity.
Next, the authors wondered what effect BACE inhibitors might have on Aη production. Adding RG7129, a discontinued BACE inhibitor from Hoffmann-La Roche, to mouse or human neuronal cultures boosted Aη-α production by about 65 percent it while dropped Aη-β, the authors found. In wild-type mice, a single dose of the BACE inhibitor doubled Aη-α three hours later. The authors saw a similar boost of Aη-α in BACE1 knockout mice, and in APP V717I transgenic mice fed the inhibitor. Co-author Jochen Herms at the German Center for Neurodegenerative Diseases, Munich, found that LTP was dampened by about a third in hippocampal slices taken from wild-type mice treated with RG7129, in line with Aη-α’s effects on synaptic plasticity. The authors did not test behavior.
The finding raises questions about the development of BACE inhibitor therapies, Haass suggested. To be clear, Haass said he is in favor of evaluating partial BACE inhibition, just not full inhibition. Several BACE inhibitors are currently in Phase 2 and 3 trials (see Dec 2013 news; Oct 2014 news). None are dosed to block BACE 100 percent. Other researchers agreed that trials would be well advised to monitor for the presence of Aη fragments in CSF, but also pointed to large existing safety data sets of the currently used inhibitors at higher doses than those used in the trials. Selkoe recommended that CSF from people undergoing treatment be analyzed by mass spectrometry, to track changes in the relative abundance of numerous APP fragments.
Selkoe emphasized that it would be premature to assume that the findings constitute a major red flag for inhibitor therapy. “None of this data persuades me that BACE inhibition is a bad idea. I think it’s still a reasonable target,” Selkoe told Alzforum. He pointed out that clinicians are already titrating inhibitor dose to try to avoid too much suppression of other BACE substrates. Among dozens of BACE substrates, crucial ones include neuregulin and CHL1 (see Jul 2006 conference news; Dec 2013 conference news; Aug 2015 news).
Bitan noted that the naturally occurring Icelandic mutation in APP lowers BACE cleavage and protects against Alzheimer’s (see Jul 2012 news). This would argue against a boost in Aη-α playing a major role in human disease, as would the fact that Aη-α falls with age in mice, Bitan suggested. For BACE inhibitor trials, he said it is too early to know if Aη-α could spell trouble. “We’ll have to wait and see the data,” Bitan said.

η-CTF Loiters Outside Plaques.
In hippocampi of transgenic mice, researchers found η-CTF fragments (green) in dystrophic neurites surrounding amyloid deposits (red), but not in plaque cores themselves. [Courtesy of Willem et al., Nature.]
The data leave open the question of whether Aη fragments contribute to Alzheimer’s pathology. Using antibodies to C-terminal and N-terminal epitopes of η-CTF, Haass and colleagues confirmed the presence of this CTF in dystrophic neurites around plaques in 6-month-old APPPS1 mice, but not in plaque cores (see image at left). They found a similar pattern in human postmortem brain tissue. However, this proximity does not necessarily indicate a role in pathology, Selkoe said, pointing out that APP and its fragments abound in neurites but often do not correlate with local Aβ deposition. Haass told Alzforum that Aη fragments do not aggregate, suggesting that they do not contribute to plaque formation.
Could they play some other role in disease? The accumulation of η-CTF in dystrophic neurites around plaques suggests the pathway may be locally induced, Haass noted. In future work, Willem will assess whether the presence of Aβ might pump up MT5-MMP activity. If so, increased Aη-α around plaques might act as a downstream effector in Alzheimer’s, contributing to the silencing of neuronal activity late in disease, he speculated.
Portelius agrees that a potential role in disease deserves further consideration. The NTE peptides his group identified in CSF, which all end at the α-secretase site, might represent pieces or degradation fragments from the Aη-α peptide, he suggested. In a pilot study, Portelius found significantly higher levels of some of the longest of these NTE α-secretase fragments in CSF from three AD patients compared to three controls, hinting at a role in disease. Haass plans to further study a possible correlation between Aη and AD by measuring CSF Aη levels in people with a familial AD mutation to see if there are consistent changes.
Overall, researchers agreed that APP processing needs further investigation. “I believe that more APP fragments remain to be discovered,” Portelius said. “We need to better understand their processing and physiological functions.”—Madolyn Bowman Rogers
References
News Citations
- Sweet 16: Novel APP Processing Pathway and a New Biomarker?
- Not the Usual Suspects: Tracking BACE Inhibition, Axon Role
- Can Dousing PyroGlu-Aβ Treat Alzheimer’s Disease?
- Do Extended Species of Aβ Poison Synapses, Masquerade As Dimers?
- Merck BACE Inhibitor Clears a Safety Hurdle, Gets New Trial
- Lilly Teams Up With AstraZeneca for BACE Inhibitor Phase 2/3 Trial
- Madrid: BACE Found to Have Big Job in Wrapping Motoneurons
- BACE—Substrates, Functions, Developmental Phenotypes
- APP Secretases Play Tug of War with Growth Cones: Could Inhibitors Dictate the Winner?
- Protective APP Mutation Found—Supports Amyloid Hypothesis
Therapeutics Citations
Research Models Citations
Paper Citations
- Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992 Jun 11;357(6378):500-3. PubMed.
- Higashi S, Miyazaki K. Novel processing of beta-amyloid precursor protein catalyzed by membrane type 1 matrix metalloproteinase releases a fragment lacking the inhibitor domain against gelatinase A. Biochemistry. 2003 Jun 3;42(21):6514-26. PubMed.
- Ahmad M, Takino T, Miyamori H, Yoshizaki T, Furukawa M, Sato H. Cleavage of amyloid-beta precursor protein (APP) by membrane-type matrix metalloproteinases. J Biochem. 2006 Mar;139(3):517-26. PubMed.
- Baranger K, Marchalant Y, Bonnet AE, Crouzin N, Carrete A, Paumier JM, Py NA, Bernard A, Bauer C, Charrat E, Moschke K, Seiki M, Vignes M, Lichtenthaler SF, Checler F, Khrestchatisky M, Rivera S. MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer's disease. Cell Mol Life Sci. 2016 Jan;73(1):217-36. Epub 2015 Jul 23 PubMed.
- Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B, Konnerth A. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2012 May 29;109(22):8740-5. Epub 2012 May 16 PubMed.
Other Citations
Further Reading
News
- APP in Pieces: βCTF implicated in Endosome Dysfunction
- New Wrinkle in APP Processing: Does C-Terminus Increase Tau Pathology?
- Partners in Crime: APP Fragment and Endosomal Protein Impair Endocytosis
- Sorting Out SorLA—What Role in APP Processing, AD?
- Double Paper Alert—A Function for BACE, a Basis for Amyloid
- Paper Alert: BACE1 Required for Muscle Spindle, Motor Control
Primary Papers
- Willem M, Tahirovic S, Busche MA, Ovsepian SV, Chafai M, Kootar S, Hornburg D, Evans LD, Moore S, Daria A, Hampel H, Müller V, Giudici C, Nuscher B, Wenninger-Weinzierl A, Kremmer E, Heneka MT, Thal DR, Giedraitis V, Lannfelt L, Müller U, Livesey FJ, Meissner F, Herms J, Konnerth A, Marie H, Haass C. η-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature. 2015 Oct 15;526(7573):443-7. Epub 2015 Aug 31 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Northwestern University Feinberg School of Medicine
The discovery of η-secretase processing of APP is an intriguing finding that may have important implications for BACE inhibition as a therapeutic approach for AD. Willem and colleagues convincingly show that BACE inhibition increases the Aη-α fragment in vitro and in vivo, and furthermore demonstrate that Aη-α impairs hippocampal LTP and reduces neuronal activity. Aη-β, which is shorter than Aη-α at the C-terminus by only 16 amino acids, does not appear to have negative effects on LTP or neuronal activity; it would be very interesting to determine why this is the case. It will also be important to characterize η-secretase processing of APP in humans and how BACE inhibition affects the balance between Aη-α and Aη-β in individuals subjected to BACE inhibition. Clearly, this is an important new area of AD research that requires further investigation. We, as a field, should pay close attention to it as we move forward with BACE inhibitors in clinical trials.…More
Institut de Pharmacologie Moléculaire et Cellulaire
The paper by Willem et al. is an interesting study on APP processing, showing that current canonical views about APP-related cleavage events are likely a very partial understanding of the overall process. It shows that an additional cleavage, occurring upstream of the BACE-1-dependent proteolysis, generates an η-CTF fragment. This η cleavage is mediated by a matrixine named MT5-MMP. This set of data are in line with our recent study performed with Dr. Santiago Rivera and colleagues (Baranger et al., 2015) showing that MT5-MMP behaved as a new pro-amyloidogenic APP-cleaving enzyme generating an sAPP95 N-terminal fragment that could well correspond to the N-terminal counterpart of η-CTF. One interesting point of Willem’s study is that η-CTF generated by MT5-MMP-mediated primary cleavage undergoes subsequent secondary proteolysis by β- and α-secretases, yielding Aη-β and Aη-α, respectively. Interestingly, only the latter can trigger hippocampal defects, illustrated by altered LTP. This agrees very well with our data showing that knocking out MT5-MMP in 5XTg mice restores LTP to normal.…More
BACE-1 blockade is reported to enhance η-CTF and Aη-α, and to lower hippocampal LTP in mice. The latter set of data challenges the notion that BACE1 inhibition rescues Alzheimer’s-like pathology and cognitive defects in transgenic animals and, at a glance, is at odds with a pathogenic Aη-α production enhanced upon BACE-1 blockade. Furthermore, it questions current views on secretase functions and raises several puzzling questions. Is β-secretase-mediated cleavage of APP beneficial and α-secretase cleavage deleterious? Is η-CTF, per se, an APP product of a pathogenic processing pathway, or is it just a precursor of subsequent toxic fragments including Aη-α, or is it both? Our previous work did not address this question, but clearly demonstrated that depletion of endogenous MT5-MMP-mediated cleavage not only improved amyloid pathology but also ameliorated hippocampal currents, inflammation stigmata, and cognitive deficits. These paradigms were not addressed in Willem and colleagues' paper, but should be examined by means of constructs expressing each of the putative pathogenic triggers linked to η-secretase cleavage and subsequent products. This is of utmost importance for both the fundamental understanding of APP processing and for an industry committed to selective and potent inhibitors directed against BACE-1.
References:
Baranger K, Marchalant Y, Bonnet AE, Crouzin N, Carrete A, Paumier JM, Py NA, Bernard A, Bauer C, Charrat E, Moschke K, Seiki M, Vignes M, Lichtenthaler SF, Checler F, Khrestchatisky M, Rivera S. MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer's disease. Cell Mol Life Sci. 2016 Jan;73(1):217-36. Epub 2015 Jul 23 PubMed.
CNRS/Aix-Marseille University
This interesting study by Willem and colleagues reinforces data we published in July (Baranger et al., 2015). Our study, a collaboration with Drs. Checler and Vignes (France), Lichtenthaler (Germany), and Seiki (Japan), shows that knockout of MT5-MMP in the 5xFAD model drastically drops the levels of Aβ and the C99 APP C-terminal fragment derived from β-secretase cleavage. MT5-MMP deletion in our bigenic mice attenuates neuroinflammation and prevents LTP dysfunction and learning deficits.
The results of Willem and colleagues in cultured neurons provide an additional mechanistic explanation for our in vivo observation: the generation of a CTFη fragment of ~30 kDa that is further processed by α- or β-secretase to generate in the first case a neurotoxic peptide Aη-α that interferes with LTP and synaptic activity. In contrast, the β-secretase-derived Aη-β product would be innocuous. This raises a provocative question on whether the activity of α-secretase would be detrimental upon BACE inhibition.…More
In our study, we do not consistently detect the ~30 kDa fragment, but we detect a 6E10+ ~12 kDa band that may represent a trimer of Aβ. Alternatively, we suggested that this band could correspond to an N-terminally elongated Aβ form cleaved by MT5-MMP and secretases. Whether the ~12 kDa band we observe is the Aη-α fragment reported in the Willem paper needs to be further evaluated. In any case, it turns out to be dramatically decreased in the brains of our bigenic 5xFAD/MT5-MMP-/- mice, indicating a clear effect of MT5-MMP in vivo. Further evidence that MT5-MMP may process APP in vivo stems from the decrease of the 90 kDa N-terminal soluble APP form that we observed in bigenic 5xFAD/MT5-MMP-/- mice, and which was reported by Willem and colleagues to migrate around 80 kDa.
Together, the Baranger and the Willem studies independently highlight the importance of MT5-MMP as an APP-processing enzyme with potential pathophysiological and translational relevance in Alzheimer’s disease. On one hand, our data demonstrate that the improved pathophysiological outcome upon MT5-MMP deficiency in AD mice occurs without alterations in the activities of β- and γ-secretases. This is important to the prospect of achieving “safe” pharmacological inhibition of MT5-MMP without the side effects commonly associated with the inhibition of β- and γ-secretases. On the other hand, the detection of the Aη-α neurotoxic fragment in CSF and brain reported by Willem and colleagues provides a biomarker for indirect monitoring of MT5-MMP activity, as well as a potential therapeutic target on its own.
From the above it follows that modulating the activity of MT5-MMP or other MT-MMPs that share the cleavage site on APP (see our recent paper on MT1-MMP, Py et al., 2014) may open the way to designing complementary therapeutic strategies to those currently being developed. However, a necessary prerequisite is to have comprehensive knowledge of MT5-MMP physiological activities, its substrates, and its molecular interactions with key elements of the amyloidogenic cascade.
References:
Baranger K, Marchalant Y, Bonnet AE, Crouzin N, Carrete A, Paumier JM, Py NA, Bernard A, Bauer C, Charrat E, Moschke K, Seiki M, Vignes M, Lichtenthaler SF, Checler F, Khrestchatisky M, Rivera S. MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer's disease. Cell Mol Life Sci. 2016 Jan;73(1):217-36. Epub 2015 Jul 23 PubMed.
Py NA, Bonnet AE, Bernard A, Marchalant Y, Charrat E, Checler F, Khrestchatisky M, Baranger K, Rivera S. Differential spatio-temporal regulation of MMPs in the 5xFAD mouse model of Alzheimer's disease: evidence for a pro-amyloidogenic role of MT1-MMP. Front Aging Neurosci. 2014;6:247. Epub 2014 Sep 18 PubMed.
Make a Comment
To make a comment you must login or register.