EMIF, GAAIN: Online Gateways to Reams of Alzheimer’s Data
Quick Links
Alzheimer’s researchers are often hamstrung by a lack of study participants and funds. Take Simon Lovestone at Oxford University. He had identified potential fluid markers of brain amyloid in small studies of cognitively normal people and wanted to confirm his finding in a sample of 1,000, but lacked the funding to assemble and follow such a cohort. Instead, Lovestone turned to an online catalog of existing cohorts maintained by the European Medical Information Framework for Alzheimer’s Disease (EMIF-AD). Lovestone coordinates EMIF along with Bart Vannieuwenhuyse at Janssen Pharmaceuticals, Beerse, Belgium. The catalog allowed Lovestone to identify participants in several different studies who had the characteristics he was looking for in terms of age, sex, cognitive status, amyloid PET data, and availability of fluid samples. Through EMIF-AD, he then contacted the administrators of each study and requested access to the raw data and fluid samples. Fluid aliquots were shipped to Oxford, and presto, Lovestone quickly and inexpensively assembled a large, ad hoc cohort. He is now analyzing that data. He believes this approach overcomes a major problem of Alzheimer’s research. “We are no longer limited by sample availability,” Lovestone told Alzforum.
His approach may become more common. Growing numbers of Alzheimer’s and aging studies make their data available through EMIF-AD and a similar effort based in the United States called the Global Alzheimer’s Association Interactive Network (GAAIN). Both are large, multinational initiatives that provide online “one-stop shops” for human research data. Each collates data from large numbers of observational or therapeutic human studies, with GAAIN extending worldwide and EMIF-AD focusing on European research. Besides using GAAIN and EMIF-AD to identify cohorts that have the data needed to address specific research questions, researchers can also use these resources to perform certain meta-analyses of data across multiple cohorts. In cases where research participants have granted permission to be re-contacted, researchers can use the catalog to find people interested in taking part in future studies. Scientists in the field believe GAAIN and EMIF-AD will foster data and sample sharing, support trial recruitment, and speed the pace of research.
“Researchers can complete queries of metadata very quickly to determine if they want to make a formal request for raw data and samples. Having this curated, single access point to find out what data and samples are accessible will accelerate analyses, and planning of additional studies,” explained Randall Bateman at Washington University School of Medicine in St. Louis in an email to Alzforum. He leads the Dominantly Inherited Alzheimer Network (DIAN), which makes its data available through GAAIN.
The respective sizes of the databases provide great statistical power to find small differences between controls and people on the path to AD, noted Arthur Toga at the University of Southern California, Los Angeles, who leads GAAIN. GAAIN currently compiles data on some 400,000 participants in aging and AD studies, while EMIF comprises about 60,000. “GAAIN becomes a powerful tool to see whether biological signals of interest hold across different cohorts and across the variations in the methodology they use,” Toga told Alzforum. Pieter-Jelle Visser at VU University Medical Centre, Amsterdam, who works on EMIF-AD, agreed. “Everybody understands that the bigger the data sets, the better the analysis,” he said.
GAAIN and EMIF-AD share certain features. Both leave control of raw data in the hands of the individual cohort investigators, while permitting users to search general descriptions of what is available. Both allow users to run some cross-cohort analyses from a central website. The two initiatives employ different architecture, however, and they collate largely distinct data sets with only a few cohorts in common. The EMIF-AD software also has been adapted for use by other European projects, such as the European Prevention of Alzheimer’s Disease (EPAD) trial platform and the Dementias Platform UK (see Aug 2016 conference news). In addition, EMIF-AD funds new research, including a biomarker study on identical twins that will facilitate dissection of the genetic contribution to disease.
EMIF-AD Spurs Research
EMIF launched in 2013 as a public-private partnership funded by a five-year grant from the Innovative Medicines Initiative with equal support from the EU and pharmaceutical partners. The goal was to build online infrastructure to support data sharing. Components included a catalog of existing cohort studies, a software platform that facilitates data analysis and includes some of the raw cohort data, and a database of electronic health registry records across Europe. Initially, EMIF focused these resources on two research areas: Alzheimer’s and the metabolic consequences of obesity.
AD researchers interested in using the resource can start by searching the online catalog. Users currently must submit a request to join the EMIF community, but in the future the catalog will be open to the public, noted Lovestone. The catalog currently lists 45 cohorts, mostly drawn from 14 European countries, including the Amsterdam Dementia Cohort, the DESCRIPA biomarker study, the drug discovery program Pharma-Cog, and the FINGER prevention trial (see Mar 2013 news; Nov 2015 conference news). The U.S.-based ADNI study is included as well.
| COHORT | EMIF-AD | GAAIN |
|---|---|---|
| AddNeuroMed | X | |
| ADNI | X | X |
| AgeCoDe | X | |
| AIBL | X | |
| Amsterdam Dementia Cohort | X | |
| Antwerp Cohort | X | |
| Alzheimer’s Disease Repository Without Borders | X | X |
| Athens Cohort | X | |
| BARCELONA-SANT PAU | X | |
| BIOCARD | X | |
| CAIDE | X | |
| CAMD | X | |
| CLSA | X | |
| Cog-Laus | X | |
| Dementia and Aging Research of Taiwan (DART) | X | |
| Dementia Case Register | X | |
| DESCRIPA | X | |
| DESCRIPA population cohort | X | |
| DIAN | X | |
| DiMI | X | |
| Donepezil | X | |
| EADC-PET | X | |
| EADC prodromal | X | |
| EDAR | X | |
| European Diffusion Tensor Imaging Study in Dementia (EDSD) | X | |
| FINGER | X | |
| Framingham Heart Study | X | |
| French National Alzheimer Database | X | |
| Fundació ACE | X | |
| Proyecto Gipuzkoa Alzheimer (GAP) | X | |
| Gothenburg MCI | X | |
| Health and Retirement Study | X | |
| HELIAD | X | X |
| IDIBAPS | X | |
| IMAP | X | |
| Italian ADNI | X | |
| Layton Aging and Alzheimer’s Disease Center | X | |
| Laboratory of Magnetic Resonance Research (LMRR) | X | |
| LeARN | X | |
| Leuven 1 | X | |
| Kuopio Longitudinal MCI Study | X | |
| Memento Cohort | X | |
| Milan Cohort | X | |
| MRC-CFAS | X | |
| National Alzheimer’s Coordinating Center | X | |
| NEST-DD | X | |
| NIAGADS | X | |
| OASIS | X | |
| Oslo Cohort | X | |
| PharmaCog | X | X |
| Prediction of Alzheimer’s Disease | X | |
| Parelsnoer Institute | X | |
| Heinz Nixdorf Recall Study | X | |
| Rete Geriatrica Alzheimer Italian | X | |
| SNAC-K | X | |
| UPenn’s Integrated Neurodegenerative Disease Database | X | |
| Wisconsin Longitudinal Study | X | |
| WRAP | X |
Researchers can peruse summary data from each cohort, including such factors as participants’ cognitive status, what clinical and cognitive tests they have taken, and what imaging, fluid biomarker, and genotyping data are available. Summary data is displayed in the form of a questionnaire filled out by study investigators. To access specific findings or biological samples, researchers submit a request through the website to the cohort curators. Once the request is granted, curators can upload the data onto a software platform called tranSMART for analysis.
To date, nine of the 45 cohorts, comprising more than 3,000 participants, have provided their full data for tranSMART. Findings are stripped of identifying information and harmonized so that data can be pooled and compared between different cohorts. This allows researchers to compare “apples to apples,” Vannieuwenhuyse noted. The data standardization distinguishes EMIF-AD from GAAIN, which does not have an analogous feature.
Only registered users can access the database. They can examine about 50 variables, including demographic information, imaging results, fluid biomarkers, cognitive test scores, and clinical data, as well as genomic, proteomic, and metabolomic findings. The tranSMART platform allows researchers to run analyses and export data, as well.
Numerous research groups are drawing on the EMIF-AD resources to perform large-scale analyses. Some of these are published, including a meta-analysis of the prevalence of amyloid pathology in more than 7,500 cognitively normal older adults, and an evaluation of rates of progression to AD in groups of people with mild cognitive impairment (see May 2015 news on Jansen et al., 2015; Vos et al., 2015). Other studies are developing risk scores for dementia prediction, and determining normative cognitive test scores in people with or without brain amyloid, Visser said. Some projects, such as Lovestone’s biomarker analysis of existing fluid samples, generate new proteomic and metabolomic data that will enrich existing cohort data, Vannieuwenhuyse noted (see Voyle et al., 2016; Westwood et al., 2016). Lovestone suggested that this could initiate new areas for drug discovery. “I’m excited by the prospect of being able to move between huge clinical data sets to molecular data,” he told Alzforum.
Stephanie Vos at Maastricht University, the Netherlands, participates in the development of the EMIF catalog and database and has used them for research. Vos wrote, “Having a common place for information on AD cohorts makes it easier to find the appropriate data for your study and set up collaborations. Its future utility will largely depend on the data owners, as they are responsible for updating information about their cohorts.”
GAAINing Ground
Launched in 2015 and supported by the National Institutes of Health and the Alzheimer’s Association, GAAIN currently provides access to 24 data sets (see also Toga et al., 2016). Besides major cohorts in AD research, such as AIBL, ADNI, and DIAN, these include huge data sets like NACC, NIAGADS, and the Framingham Heart Study. GAAIN calls these participating studies “data partners.” While most are in North America, GAAIN also includes a smattering from Europe, Australia, and Taiwan.
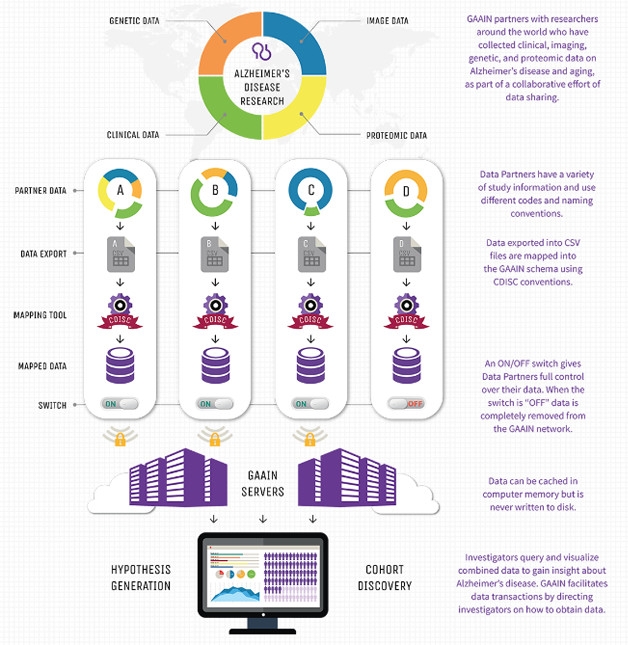
Separate Data, One Portal. GAAIN’s decentralized system allows users to search data maintained on individual investigator servers. [Courtesy of Arthur Toga.]
More cohorts from around the world are in the process of coming on board, said Toga, who is aiming for a total of at least 170 partners. Upcoming data partners include the Alzheimer’s Prevention Initiative, run by Eric Reiman and Pierre Tariot at Banner Alzheimer’s Institute, Phoenix. “API is excited about the chance to participate in GAAIN. We believe that user-friendly mechanisms to share data and biological samples will help to inform the design and power of prevention trials and find better ways to evaluate the range of promising but unproven treatments,” Reiman wrote to Alzforum.
GAAIN partners extend throughout the U.S., Europe, and Australia. [Courtesy of Arthur Toga.]

Worldwide Reach.
GAAIN also provides the ability to analyze data across cohorts, using its “Interrogator” tool. Once users complete a free registration step, they can look for correlations among a handful of variables across multiple cohorts and view the results in graph form. The variables include imaging and fluid biomarker data, demographic information, ApoE genotype, and cognitive test results. To download results of the analysis, or to request the raw data, users must abide by the data use requirements of each partner site.
Toga told Alzforum that use of the online resources has surged, with more than 100 logins per day on the Interrogator tool alone, although to date there are no published studies that cite GAAIN data. Toga believes one benefit of examining research across cohorts is that holes in the data become apparent. “We can use this as a tool to understand the entire landscape of Alzheimer’s disease research and identify places where additional investigation needs to be undertaken. That will be instrumental in advancing our knowledge faster. This is the wave of the future,” he said. Toga himself has used GAAIN to investigate how risk factors for AD vary between men and women, and how taking statins affects risk. Those data will be published soon, but in a nutshell, Toga found that statins exacerbate AD risk among women but not men.
GAAIN can also facilitate collaborative research. Scientists who are developing the centiloid scale to standardize amyloid imaging data have used GAAIN to post their reference scans, so that other researchers can harmonize their own data against them (see Nov 2014 news).
Electronic Health Records: Leveraging Quantity over Quality
While EMIF and GAAIN are similar in terms of providing access to AD cohort data, EMIF includes some unique features. In addition to cohort data, EMIF compiles nine large electronic health records (EHR) data sets from across Europe. These comprise clinical data from hospitals, nursing homes, primary care clinics, and health registries, covering 50 million adults and children. While the database is vast, data quality can vary. At the 2016 AAIC conference in Toronto, Robert Stewart of King’s College London illustrated both the power and the pitfalls of this type of data. Stewart extracted information on dementia diagnoses, year of diagnosis, and year of death from six of the EHRs in the database, totaling several million people. About 140,000 of them had been diagnosed with dementia. A meta-analysis found steadily increasing prevalence over the decades, as expected, while incidence increased only in the oldest age groups.
When Stewart compared his findings to estimates of prevalence and incidence from community studies, however, the results did not match. Prevalence estimated from the EHRs was about one-third of that from community studies, while incidence ran about half of that previously reported. The findings imply that health professionals in non-specialist settings underdiagnose dementia at mild stages, leading to lower estimates in medical records than in aging studies, Stewart suggested. Other researchers pointed out that many population studies report declining incidence over the last two decades, which did not show up in the EHR data (see Feb 2016 news; Apr 2016 news). Stewart agreed that electronic health records have gaps because people may not go to hospitals or nursing homes until they are quite ill, and thus the records could miss key trends like this.
New Biomarker Data May Reveal Genetic Contributions
In addition to compiling previous research results, EMIF-AD also funds new studies. For example, the researchers have started the EMIF-AD 90+ study, which will enroll 120 nonagenarians and centenarians, some cognitively normal and some with dementia, in search of resilience factors. In December 2014, EMIF began collecting longitudinal biomarker data from 300 cognitively normal older adults, including 97 identical twin pairs and six single twins, for the EMIF-AD PreclinAD study. Amyloid burden is highly correlated among twins, suggesting a strong genetic component to accumulation. The researchers are also measuring brain connectivity and vascular health. Twin data intrigue researchers because of their potential to disentangle genetic and environmental contributions to disease.

A twin pair in EMIF-AD preclinical Alzheimer’s research. [Courtesy of Pieter Jelle Visser.]
Anouk den Braber of VU University, Amsterdam, has analyzed structural MRI data from 72 of the twin pairs. Their average age was 69; they came from the Netherlands. Den Braber focused on cortical thinning, which accelerates as people develop AD. She measured an average correlation of 0.67 in the cortical thickness of brain regions susceptible to dementia between identical twins. Cortical thickness does not correlate among randomly paired people. This suggests that two-thirds of cortical thickness is determined by genetics, leaving about one-third to be influenced by environment and lifestyle, den Braber concluded. Researchers have found other intriguing results from twins as well. Visser is studying a pair in which one twin has dementia and a brain packed with amyloid, while the other remains cognitively normal and largely amyloid-free. He is looking for a possible de novo genetic abnormality that might explain this by analyzing the whole genomes of each twin.
Overall, researchers hope the resources available through GAAIN and EMIF will accelerate research. A big roadblock for AD researchers is recruitment for studies (see Nov 2013 news), but GAAIN and EMIF now provide ready access to a vast number of studies and samples, so for certain questions, scientists may not need to recruit at all. Programs such as EPAD are using the databases to identify potential participants for clinical trials.
GAAIN and EMIF operate separately from each other, and at the moment have no plans to make their respective data sets analyzable as one. In the future, however, researchers may be able to compare data obtained from GAAIN and EMIF-AD. “We are working toward a collaborative process so that we can translate from one to the other,” Lovestone said. Toga noted that these initiatives will further develop the social community of AD research.—Madolyn Bowman Rogers
References
News Citations
- Coming to a Center Near You: GAP and EPAD to Revamp Alzheimer’s Trials
- More Bang for the Buck? DESCRIPA Thrifty in Biomarker Studies
- Health Interventions Boost Cognition—But Do They Delay Dementia?
- Meta-Analyses Deliver Most Definitive Data Yet on Amyloid Prevalence
- Paper Alert: Centiloid Scale Aims to Unify Amyloid PET
- Falling Dementia Rates in U.S. and Europe Sharpen Focus on Lifestyle
- Dementia Incidence in Britain Dropped, Mostly in Men
- A Conference Devoted to Better Engaging Clinical Trial Volunteers
Paper Citations
- Dubois B, Chupin M, Hampel H, Lista S, Cavedo E, Croisile B, Tisserand GL, Touchon J, Bonafe A, Ousset PJ, Ait Ameur A, Rouaud O, Ricolfi F, Vighetto A, Pasquier F, Delmaire C, Ceccaldi M, Girard N, Dufouil C, Lehericy S, Tonelli I, Duveau F, Colliot O, Garnero L, Sarazin M, Dormont D, “Hippocampus Study Group”. Donepezil decreases annual rate of hippocampal atrophy in suspected prodromal Alzheimer's disease. Alzheimers Dement. 2015 Jan 14; PubMed.
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ, Amyloid Biomarker Study Group, Aalten P, Aarsland D, Alcolea D, Alexander M, Almdahl IS, Arnold SE, Baldeiras I, Barthel H, van Berckel BN, Bibeau K, Blennow K, Brooks DJ, van Buchem MA, Camus V, Cavedo E, Chen K, Chetelat G, Cohen AD, Drzezga A, Engelborghs S, Fagan AM, Fladby T, Fleisher AS, van der Flier WM, Ford L, Förster S, Fortea J, Foskett N, Frederiksen KS, Freund-Levi Y, Frisoni GB, Froelich L, Gabryelewicz T, Gill KD, Gkatzima O, Gómez-Tortosa E, Gordon MF, Grimmer T, Hampel H, Hausner L, Hellwig S, Herukka SK, Hildebrandt H, Ishihara L, Ivanoiu A, Jagust WJ, Johannsen P, Kandimalla R, Kapaki E, Klimkowicz-Mrowiec A, Klunk WE, Köhler S, Koglin N, Kornhuber J, Kramberger MG, Van Laere K, Landau SM, Lee DY, de Leon M, Lisetti V, Lleó A, Madsen K, Maier W, Marcusson J, Mattsson N, de Mendonça A, Meulenbroek O, Meyer PT, Mintun MA, Mok V, Molinuevo JL, Møllergård HM, Morris JC, Mroczko B, Van der Mussele S, Na DL, Newberg A, Nordberg A, Nordlund A, Novak GP, Paraskevas GP, Parnetti L, Perera G, Peters O, Popp J, Prabhakar S, Rabinovici GD, Ramakers IH, Rami L, Resende de Oliveira C, Rinne JO, Rodrigue KM, Rodríguez-Rodríguez E, Roe CM, Rot U, Rowe CC, Rüther E, Sabri O, Sanchez-Juan P, Santana I, Sarazin M, Schröder J, Schütte C, Seo SW, Soetewey F, Soininen H, Spiru L, Struyfs H, Teunissen CE, Tsolaki M, Vandenberghe R, Verbeek MM, Villemagne VL, Vos SJ, van Waalwijk van Doorn LJ, Waldemar G, Wallin A, Wallin ÅK, Wiltfang J, Wolk DA, Zboch M, Zetterberg H. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015 May 19;313(19):1924-38. PubMed.
- Vos SJ, Verhey F, Frölich L, Kornhuber J, Wiltfang J, Maier W, Peters O, Rüther E, Nobili F, Morbelli S, Frisoni GB, Drzezga A, Didic M, van Berckel BN, Simmons A, Soininen H, Kłoszewska I, Mecocci P, Tsolaki M, Vellas B, Lovestone S, Muscio C, Herukka SK, Salmon E, Bastin C, Wallin A, Nordlund A, de Mendonça A, Silva D, Santana I, Lemos R, Engelborghs S, Van der Mussele S, Alzheimer’s Disease Neuroimaging Initiative, Freund-Levi Y, Wallin ÅK, Hampel H, van der Flier W, Scheltens P, Visser PJ. Prevalence and prognosis of Alzheimer's disease at the mild cognitive impairment stage. Brain. 2015 May;138(Pt 5):1327-38. Epub 2015 Feb 17 PubMed.
- Voyle N, Kim M, Proitsi P, Ashton NJ, Baird AL, Bazenet C, Hye A, Westwood S, Chung R, Ward M, Rabinovici GD, Lovestone S, Breen G, Legido-Quigley C, Dobson RJ, Kiddle SJ, Alzheimer's Disease Neuroimaging Initiative. Blood metabolite markers of neocortical amyloid-β burden: discovery and enrichment using candidate proteins. Transl Psychiatry. 2016 Jan 26;6:e719. PubMed.
- Westwood S, Leoni E, Hye A, Lynham S, Khondoker MR, Ashton NJ, Kiddle SJ, Baird AL, Sainz-Fuertes R, Leung R, Graf J, Hehir CT, Baker D, Cereda C, Bazenet C, Ward M, Thambisetty M, Lovestone S. Blood-Based Biomarker Candidates of Cerebral Amyloid Using PiB PET in Non-Demented Elderly. J Alzheimers Dis. 2016 Mar 29;52(2):561-72. PubMed.
- Toga AW, Neu SC, Bhatt P, Crawford KL, Ashish N. The Global Alzheimer's Association Interactive Network. Alzheimers Dement. 2016 Jan;12(1):49-54. Epub 2015 Aug 28 PubMed.
External Citations
- European Medical Information Framework for Alzheimer’s Disease
- Global Alzheimer’s Association Interactive Network
- European Prevention of Alzheimer’s Disease
- Dementias Platform UK
- Pharma-Cog
- AddNeuroMed
- ADNI
- AIBL
- BIOCARD
- CAIDE
- CAMD
- CLSA
- Cog-Laus
- Dementia and Aging Research of Taiwan (DART)
- DESCRIPA
- DIAN
- DiMI
- EADC-PET
- FINGER
- Framingham Heart Study
- French National Alzheimer Database
- Fundació ACE
- Proyecto Gipuzkoa Alzheimer
- Health and Retirement Study
- HELIAD
- IDIBAPS
- IMAP
- Italian ADNI
- Layton Aging and Alzheimer’s Disease Center
- LeARN
- Memento Cohort
- MRC-CFAS
- National Alzheimer’s Coordinating Center
- NEST-DD
- NIAGADS
- OASIS
- Parelsnoer Institute
- SNAC-K
- Wisconsin Longitudinal Study
- WRAP
- Interrogator
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.