ELOVL Hurts—Enzyme Makes Lipids That Turn Astrocytes Toxic
Quick Links
Astrocytes support neurons in the brain but occasionally turn on them, releasing a long-sought mystery substance that kills not only neurons but oligodendrocytes as well. More than 10 years after discovering this phenomenon, scientists still do not know the nature of this neurotoxin. Now, in the October 6 Nature, researchers led by Shane Liddelow, New York University—tipping their hat to their co-author, the late and great Ben Barres—propose that it is not a protein, as had been suspected all along. It's a lipid, they say. To be precise, it's a series of long-chain saturated fatty acids.
- Under certain conditions, astrocytes can kill.
- Their weapon: certain long-chain saturated fatty acids.
- Knocking out a lipid elongase dulls these reactive astrocytes.
These lipids assemble with ApoE or ApoJ into toxic lipoprotein particles, the authors report. Exactly how these particles damage cells remains to be seen, but knocking out ELOVL1, the elongase responsible for building these lipids, rendered reactive astrocytes much less toxic. “A lipid toxin is not what we expected, but we think it is a fascinating finding and could open up a host of new research avenues,” Liddelow told Alzforum. “ELOVL1 inhibitors would be a first-in-class therapeutic that might be helpful in treatment of a range of neurodegenerative disorders,” he said.
“This is very interesting work unraveling a long-standing secret behind astrocyte-driven neurotoxicity,” wrote Jonathan Kipnis, Washington University, St. Louis. “Saturated lipids identified in this new study may be the mediators of astrocyte toxicity in AD and Rett syndrome as well as in other neurological diseases.”
“It is very elegant work, and the data on oligodendrocytes seems quite compelling,” said Serge Przedborski, Columbia University, New York. “More work is needed before we can be comfortable that what they see in oligodendrocytes reflects faithfully the toxicity others have shown in neurons,” he added.
In 2007, Przedborski and colleagues reported that, under certain conditions, astrocytes release a toxin that kills motor neurons, indicating that this mechanism might operate in amyotrophic lateral sclerosis and other neurodegenerative diseases. Researchers in Kevin Eggan’s lab at Harvard University reported similar findings around the same time (April 2007 news). Since then, many scientists, including Barres, have been on the hunt for this toxin.
Ten years later, while working in Barres’ lab at Stanford University, Liddelow found that microglia can force astrocytes into a reactive state that he called A1 (Jan 2017 news). The label was a nod to the M1 and M2 types of microglia that researchers were focusing on at the time. While the microglial involvement complicated matters, it also helped the scientists identify a cocktail of three proinflammatory cytokines they subsequently used to generate A1 astrocytes in vitro.
For the new work, first author Kevin Guttenplan at Stanford started the groups' search for the proposed toxic factor by aggravating cultured astrocytes with this mix of C1q, Interleukin-1α, and tumor necrosis factor, and then probing the conditioned cell medium by incubating it with cultured oligodendrocytes.
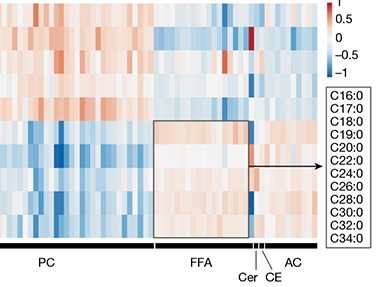
Lipids Galore. Measuring levels of different lipids (columns) revealed that conditioned medium from reactive astrocytes (bottom five rows) contains much more free fatty acids (FFA, boxed, legend indicates chain length) than does medium from control astrocytes (top five rows). Reactive cells also released more ceramides (Cer), cholesteryl esters (CE) and acylcarnitines (AC), but less phophatidylcholines (PC). [Courtesy of Guttenplan et al., Nature.]
For years, the scientists searched for potentially toxic proteins in the conditioned medium using mass spectrometry. This turned up nada. Next, Guttenplan turned to biochemical techniques to try to purify the offending molecule. This led him to fractions enriched for the apolipoproteins ApoE and ApoJ. However, conditioned media from ApoE or ApoJ knockout lines were also toxic, exonerating these two proteins.
Could the lipids that coat these lipoproteins possibly be the culprit? Indeed, removing lipids from the apolipoprotein fractions abolished toxicity, while lipids isolated from the conditioned medium were highly toxic on their own.
What kind of lipids were involved? To find out, the scientists used unbiased lipidomics. They compared levels of 1,501 lipids from 10 different classes in the conditioned medium from quiescent and reactive astrocytes. The latter contained many more long-chain saturated fatty acids, suggesting these might be the toxins (see image above). To test this, the scientist conditionally knocked out the ELOVL1 gene in mouse astrocytes. ELOVL1 encodes an elongase enzyme that lengthens saturated fatty acids containing 18 or more carbons, generating chains up to 30 carbons long (see diagram below).

Toxic Branch of the Family. ELOVL1 and related elongases extend the carbon backbone of certain saturated fatty acids, i.e., those devoid of double carbon bonds. These are different than the ω3 and ω6 saturated fatty acids people get through their diet. [Courtesy of Shane Liddelow.]
When provoked by the cytokine cocktail, astrocytes cultured from the elongase knockout brain became only weakly toxic compared to reactive astrocytes from wild-type mice, which readily killed co-cultured oligodendrocytes.
Can these free fatty acids also harm neurons? Cultured retinal ganglion cells died quickly when exposed to astrocyte conditioned medium, or to reconstituted high-density lipoproteins made by combining ApoE/J and lipids extracted from the medium. In an optical nerve crush experiment, an established in vivo model of neuronal viability after injury, many more neurons survived such an injury in ELOVL1 knockout than in control mice, implicating long-chain fatty acids in neurotoxicity (see image below).
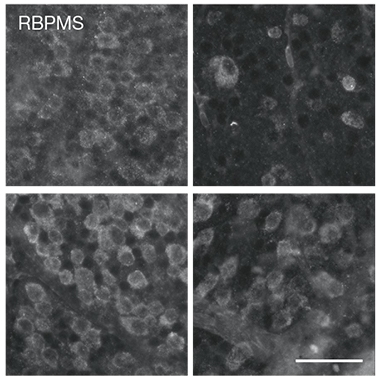
Better Optics. In control mice (top) retinal ganglion cells (left) died 14 days after the optic nerve had been crushed (right). In EVOLV1 knockout animals (bottom), more of those neurons survived the injury. [Courtesy of Guttenplan et al., Nature.]
Still, the effect was not absolute. Some of the neurons did succumb, leading the authors to conclude that astrocytes might release yet more factors that are neurotoxic. Commentators echoed this point. “Our own recent results suggest that exocytosis of remodeled lysosomes from inflammatory reactive astrocytes mediates neurotoxicity, so we are excited to test whether saturated lipids may also be released via this pathway, or whether other lysosomal contents contribute to neurotoxicity,” wrote Martin Kampmann, University of California, San Francisco (Sep 2021 news).
In Przedborski's hands, removing lipids from astrocyte conditioned medium did not abolish its toxicity to motor neurons (Mishra et al., 2020). “Testing the retinal ganglion cells is a great idea,” Przedborski said, “but we’d also like to know what is happening to motor neurons and other neurons that the field is more excited to learn about.”
Przedborski emphasized that the toxicity of astrocytes is context-dependent. This point is also raised by the authors, and explained in depth in a recent consensus paper on astrocyte terminology and research priorities (Escartin et al., 2021).
On this point, Francisco Quintana, Brigham and Women’s Hospital, Boston, stressed that other subsets of astrocytes may be neurotoxic besides those roused by the cytokine cocktail the Liddelow lab used. Indeed, by now scientists generally agree that the A1/A2 characterization of astrocytes is an oversimplification, much like they are abandoning the M1/M2 dichotomy.
Nevertheless, Quintana is excited by the data. “It may dovetail with some of the lipid metabolic remodeling we have discovered in models of neurodegeneration,” he told Alzforum. For example, Quintana and colleagues have found that lactosylceramide makes astrocytes neurotoxic and inflammatory in experimental autoimmune encephalitis, a model for multiple sclerosis (Mayo et al., 2014; Chao et al., 2019). Lactosylceramide a glycosphingolipid, sports a lactose sugar moiety tacked onto a sphingosine/fatty acid backbone that makes up ceramide.
By way of its involvement in fatty acid synthesis, ELOVL1 is linked to ceramide metabolism as well. In fact, the metabolism of many of these lipids is interrelated, noted Quintana. This made him question whether ELOVL1 can become a viable therapeutic target. “Some long-chain fatty acids have beneficial effects, so if you interfere with their synthesis, you might have deleterious effects, for example disrupting myelin integrity,” he warned.
Currently, no drugs directly targeting ELOVL1 are in clinical trials. Coenzyme A esters of benzafibrate and gemfibrozil block the elongase and reduce levels of very-long-chain fatty acids in fibroblasts taken from people who have X-linked adrenoleukodystrophy, a lipid-storage disorder (Shackmann et al., 2015; Engelen et al., 2012). Benzafibrate failed to reduce long-chain fatty acids in a clinical trial.—Tom Fagan
References
News Citations
- Glia—Absolving Neurons of Motor Neuron Disease
- Microglia Give Astrocytes License to Kill
- Reactive Astrocytes Boot Basic, Dysfunctional Lysosomes
Therapeutics Citations
Paper Citations
- Mishra V, Re DB, Le Verche V, Alvarez MJ, Vasciaveo A, Jacquier A, Doulias PT, Greco TM, Nizzardo M, Papadimitriou D, Nagata T, Rinchetti P, Perez-Torres EJ, Politi KA, Ikiz B, Clare K, Than ME, Corti S, Ischiropoulos H, Lotti F, Califano A, Przedborski S. Systematic elucidation of neuron-astrocyte interaction in models of amyotrophic lateral sclerosis using multi-modal integrated bioinformatics workflow. Nat Commun. 2020 Nov 4;11(1):5579. PubMed.
- Escartin C, Galea E, Lakatos A, O'Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhäuser C, Volterra A, Carmignoto G, Agarwal A, Allen NJ, Araque A, Barbeito L, Barzilai A, Bergles DE, Bonvento G, Butt AM, Chen WT, Cohen-Salmon M, Cunningham C, Deneen B, De Strooper B, Díaz-Castro B, Farina C, Freeman M, Gallo V, Goldman JE, Goldman SA, Götz M, Gutiérrez A, Haydon PG, Heiland DH, Hol EM, Holt MG, Iino M, Kastanenka KV, Kettenmann H, Khakh BS, Koizumi S, Lee CJ, Liddelow SA, MacVicar BA, Magistretti P, Messing A, Mishra A, Molofsky AV, Murai KK, Norris CM, Okada S, Oliet SH, Oliveira JF, Panatier A, Parpura V, Pekna M, Pekny M, Pellerin L, Perea G, Pérez-Nievas BG, Pfrieger FW, Poskanzer KE, Quintana FJ, Ransohoff RM, Riquelme-Perez M, Robel S, Rose CR, Rothstein JD, Rouach N, Rowitch DH, Semyanov A, Sirko S, Sontheimer H, Swanson RA, Vitorica J, Wanner IB, Wood LB, Wu J, Zheng B, Zimmer ER, Zorec R, Sofroniew MV, Verkhratsky A. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021 Mar;24(3):312-325. Epub 2021 Feb 15 PubMed.
- Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, Mascanfroni ID, Yeste A, Kivisäkk P, Kallas K, Ellezam B, Bakshi R, Prat A, Antel JP, Weiner HL, Quintana FJ. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med. 2014 Oct;20(10):1147-56. Epub 2014 Sep 14 PubMed.
- Chao CC, Gutiérrez-Vázquez C, Rothhammer V, Mayo L, Wheeler MA, Tjon EC, Zandee SE, Blain M, de Lima KA, Takenaka MC, Avila-Pacheco J, Hewson P, Liu L, Sanmarco LM, Borucki DM, Lipof GZ, Trauger SA, Clish CB, Antel JP, Prat A, Quintana FJ. Metabolic Control of Astrocyte Pathogenic Activity via cPLA2-MAVS. Cell. 2019 Dec 12;179(7):1483-1498.e22. Epub 2019 Dec 5 PubMed.
- Schackmann MJ, Ofman R, Dijkstra IM, Wanders RJ, Kemp S. Enzymatic characterization of ELOVL1, a key enzyme in very long-chain fatty acid synthesis. Biochim Biophys Acta. 2015 Feb;1851(2):231-7. Epub 2014 Dec 11 PubMed.
- Engelen M, Schackmann MJ, Ofman R, Sanders RJ, Dijkstra IM, Houten SM, Fourcade S, Pujol A, Poll-The BT, Wanders RJ, Kemp S. Bezafibrate lowers very long-chain fatty acids in X-linked adrenoleukodystrophy fibroblasts by inhibiting fatty acid elongation. J Inherit Metab Dis. 2012 Nov;35(6):1137-45. Epub 2012 Mar 24 PubMed.
Further Reading
Primary Papers
- Guttenplan KA, Weigel MK, Prakash P, Wijewardhane PR, Hasel P, Rufen-Blanchette U, Münch AE, Blum JA, Fine J, Neal MC, Bruce KD, Gitler AD, Chopra G, Liddelow SA, Barres BA. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature. 2021 Nov;599(7883):102-107. Epub 2021 Oct 6 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University in St. Louis, School of Medicine
This is very interesting work unraveling a long-standing secret behind astrocyte-driven neurotoxicity. Activated astrocytes can acquire a neurotoxic phenotype, but the exact molecules that drive such neurotoxicity have been unknown. In this paper, Liddelow and colleagues have discovered the factors secreted by activated astrocytes to be saturated lipids contained in APOE and APOJ lipoparticles. Using in vitro systems, they have nicely demonstrated the specificity of the toxicity driven by these mediators.
Astrocytic apoE has been shown to increase neurodegeneration in tau models of neurodegeneration (Wang et al., 2021) and astrocytes have been shown to play a key role in Rett syndrome neurodegeneration (Lioy et al., 2011) but the actual molecules driving the neurotoxicity phenotype were unknown. Saturated lipids identified in this new study may be the mediators of astrocyte toxicity in AD and Rett syndrome as well as in other neurological diseases.
Future work should focus on examining the in vivo relevance of the saturated lipids contained in APOE and APOJ lipoparticles derived from astrocytes in different diseases.
References:
Wang C, Xiong M, Gratuze M, Bao X, Shi Y, Andhey PS, Manis M, Schroeder C, Yin Z, Madore C, Butovsky O, Artyomov M, Ulrich JD, Holtzman DM. Selective removal of astrocytic APOE4 strongly protects against tau-mediated neurodegeneration and decreases synaptic phagocytosis by microglia. Neuron. 2021 May 19;109(10):1657-1674.e7. Epub 2021 Apr 7 PubMed.
Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, Mandel G. A role for glia in the progression of Rett's syndrome. Nature. 2011 Jun 29;475(7357):497-500. PubMed.
University of California, San Francisco
In their tour de force study, Guttenplan, Liddelow and colleagues provide compelling evidence in vitro that inflammatory reactive astrocytes can release lipoparticles containing long-chain saturated fatty acids and APOE or APOJ lipoproteins, and that these particles mediate cytotoxicity of astrocyte-conditioned medium.
This work opens up many interesting questions for future investigation: Given that most experiments were based on measuring toxicity to oligodendrocytes, is toxicity of inflammatory reactive astrocytes to neurons similarly mediated by lipoparticles? An in vivo toxicity assay showed only partial rescue of neurons when the synthesis of saturated long-chain fatty acids was disrupted, suggesting that additional mechanisms of toxicity may be relevant for neurons.
Our own recent results suggest that exocytosis of remodeled lysosomes from inflammatory reactive astrocytes mediates neurotoxicity, so we are excited to test whether saturated lipids may also be released via this pathway, or whether other lysosomal contents contribute to neurotoxicity.
Make a Comment
To make a comment you must login or register.