Double Dose of Prion Variant Blocks Disease
Quick Links
Prion diseases occur when a misfolded, toxic prion binds to and morphs healthy prion proteins into toxic conformations, which then spread throughout the brain. Could this propagation be stopped? New clues come from a genetic variant found in the prion gene from some members of the Fore population, a formerly cannibalistic people in Papua New Guinea. Scientists had assumed this particular polymorphism might work like other protective variants of the prion protein, by disrupting templated misfolding. However, in the June 10 Nature, John Collinge and colleagues from University College London report that the Fore variant, a valine at position 127, seems to have a different modus operandi. If scientists can figure out what that is, they may discover a way to prevent prion diseases.
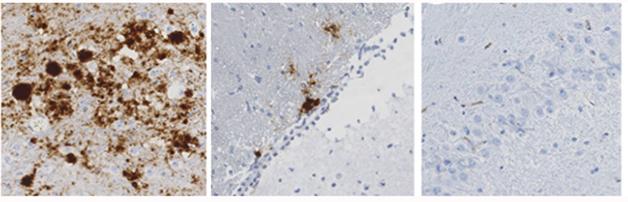
Mice inoculated with variant CJD amass PrP deposits (brown) in the brain (left), but not when they have one (middle) or two (right) copies of the protective V127 variant. [Courtesy of Asante et al., 2015. Nature.]
Decades after the 20th-century kuru epidemic, which peaked in the late 1950s, Collinge and colleagues found that a group of Fore who had been shielded from the disease carried a valine for the usual glycine at position 127 of the prion protein (see Mead et al., 2009). Carriers of this variant all had just one copy of the allele, which kept them from contracting the fatal prion disease when they ate the brains and other body parts of deceased tribe members during mourning rituals. V127 lay close to another known beneficial polymorphism in the prion protein, a methionine or valine at position 129. People who are heterozygous for this second allele are protected from prion disease, possibly because the organism produces two slightly different versions of the protein that purportedly disrupt each other's templated misfolding. When a tiny inoculum is introduced it may misfold some endogenous prion, but no explosive growth of toxic prions ensues (Palmer et al., 1991). Since protected members of the Fore population also seemed to be heterozygous for V127, the authors assumed that V127 worked the same way.
To test that hypothesis, first author Emmanuel Asante and colleagues generated mice that carried either one or two copies of V127. They then injected prion proteins from people with kuru, classical Creutzfeldt-Jacob disease (CJD), or variant CJD into the mouse brain. Classical CJD arises spontaneously or spreads via contaminated surgical instruments. The prion conformation, or strain, is the same as that for kuru, and it has been proposed that kuru came about when Fore tribesman cannibalized a person who had had sporadic CJD (Wadsworth et al., 2008). Variant CJD occurs when people eat meat from cows infected with bovine spongiform encephalopathy. This disease arises from a distinct strain of prion. Despite widespread worries in the 1990s and 2000s of an impending epidemic of human prion disease transmitted by tainted beef, vCJD has remained rare.
In this study, the researchers found that mice without the V127 allele were susceptible to all forms of prion infection (see image above). However, like the Fore population, mice that carried one copy of V127 were resistant to kuru. They also resisted classical CJD. These mice were, however, somewhat susceptible to variant CJD caused by a strain of prion to which the Fore population had never been exposed. The findings fit with the emergence of the V127 variant by natural selection against the toxic kuru/CJD strain.
However, V127’s protective effect did not seem to depend on heterozygosity. Collinge and colleagues were surprised to find that mice homozygous for V127 were completely impervious to any prion seed. This suggests that that the protective mechanism involves something other than disrupting the self-templated misfolding of endogenous prion.
“That this single amino acid change can completely prevent disease is amazing,” said David Teplow, University of California, Los Angeles. “We haven’t seen that in the prion field before.” He pointed out that while these experiments were done in transgenic mice, they may have implications for prion disease in humans. “Next we need to understand the mechanism, then we can develop therapeutic strategies based on that.” The outcome could be significant for other proteins that form amyloids, he said. Single amino acid changes in some have been shown to prevent aggregation. For instance, a mutation in the APP gene introduces a single amino acid change into the Aβ protein, protecting against AD in humans (see Di Fede et al., 2009).
“It will be important to learn what structural consequences, if any, occur when V replaces G at residue 127,” wrote Glenn Telling, Colorado State University, Fort Collins, in an accompanying News & Views article. That knowledge may hint at new ways to inhibit propagation of the toxic form of the prion protein and maintain function of the normal one, he wrote.—Gwyneth Dickey Zakaib
References
Paper Citations
- Mead S, Whitfield J, Poulter M, Shah P, Uphill J, Campbell T, Al-Dujaily H, Hummerich H, Beck J, Mein CA, Verzilli C, Whittaker J, Alpers MP, Collinge J. A novel protective prion protein variant that colocalizes with kuru exposure. N Engl J Med. 2009 Nov 19;361(21):2056-65. PubMed.
- Palmer MS, Dryden AJ, Hughes JT, Collinge J. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature. 1991 Jul 25;352(6333):340-2. PubMed.
- Wadsworth JD, Joiner S, Linehan JM, Desbruslais M, Fox K, Cooper S, Cronier S, Asante EA, Mead S, Brandner S, Hill AF, Collinge J. Kuru prions and sporadic Creutzfeldt-Jakob disease prions have equivalent transmission properties in transgenic and wild-type mice. Proc Natl Acad Sci U S A. 2008 Mar 11;105(10):3885-90. PubMed.
- Di Fede G, Catania M, Morbin M, Rossi G, Suardi S, Mazzoleni G, Merlin M, Giovagnoli AR, Prioni S, Erbetta A, Falcone C, Gobbi M, Colombo L, Bastone A, Beeg M, Manzoni C, Francescucci B, Spagnoli A, Cantù L, Del Favero E, Levy E, Salmona M, Tagliavini F. A recessive mutation in the APP gene with dominant-negative effect on amyloidogenesis. Science. 2009 Mar 13;323(5920):1473-7. PubMed.
Further Reading
Papers
- Vilette D, Laulagnier K, Huor A, Alais S, Simoes S, Maryse R, Provansal M, Lehmann S, Andreoletti O, Schaeffer L, Raposo G, Leblanc P. Efficient inhibition of infectious prions multiplication and release by targeting the exosomal pathway. Cell Mol Life Sci. 2015 Nov;72(22):4409-27. Epub 2015 Jun 6 PubMed.
- Kobayashi A, Teruya K, Matsuura Y, Shirai T, Nakamura Y, Yamada M, Mizusawa H, Mohri S, Kitamoto T. The influence of PRNP polymorphisms on human prion disease susceptibility: an update. Acta Neuropathol. 2015 Aug;130(2):159-70. Epub 2015 May 29 PubMed.
- Ning L, Pan D, Zhang Y, Wang S, Liu H, Yao X. Effects of the Pathogenic Mutation A117V and the Protective Mutation H111S on the Folding and Aggregation of PrP106-126: Insights from Replica Exchange Molecular Dynamics Simulations. PLoS One. 2015;10(5):e0125899. Epub 2015 May 20 PubMed.
- Singh J, Udgaonkar JB. Structural effects of multiple pathogenic mutations suggest a model for the initiation of misfolding of the prion protein. Angew Chem Int Ed Engl. 2015 Jun 22;54(26):7529-33. Epub 2015 May 8 PubMed.
News
- Protein Propagation Real, but Mechanisms Hazy
- A Blood Test for Preclinical Prion Disease, But Will It Be Used?
- Prion Protein Wields N-Terminal Flexible Tail to Kill Neurons
- Do Alzheimer’s and Prion Diseases Share a Pathogenic Pathway?
- Tau, α-Synuclein Spread: Crazy Stuff—How Might It Work?
- Mistaken Identity—Prion Disease or Alzheimer’s on Fast Forward?
Primary Papers
- Asante EA, Smidak M, Grimshaw A, Houghton R, Tomlinson A, Jeelani A, Jakubcova T, Hamdan S, Richard-Londt A, Linehan JM, Brandner S, Alpers M, Whitfield J, Mead S, Wadsworth JD, Collinge J. A naturally occurring variant of the human prion protein completely prevents prion disease. Nature. 2015 Jun 25;522(7557):478-81. Epub 2015 Jun 10 PubMed.
- Telling G. Neurodegeneration: Evolved protection against human prions. Nature. 2015 Jun 25;522(7557):423-4. Epub 2015 Jun 10 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.