Does Parkin Substrate Kick-Start Lewy Bodies?
Quick Links
Mutations in both parkin and α-synuclein are known to cause Parkinson’s disease, but whether their paths are intertwined is unclear. Now, researchers led by Yunjong Lee at Sungkyunkwan University School of Medicine, South Korea, suggest they are indeed connected. In the November 11 Science Translational Medicine, Lee’s group reported that the parkin substrate AIMP2 itself forms aggregates in brain cells and in mouse models. These seed the formation of α-synuclein inclusions. When triggered by oxidative stress or α-synuclein aggregates themselves, AIMP2 clumped together and stuck to α-synuclein, prompting a vicious cycle of aggregation, according to the researchers. Getting rid of AIMP2 reduced or prevented α-synuclein aggregation and saved dopaminergic neurons. If this relationship holds true, it would be a molecular pathway between parkin, α-synuclein, and Lewy bodies.
- Oxidative stress causes AIMP2 to aggregate.
- AIMP2 and α-synuclein found in Lewy bodies in PD tissue.
- Knocking down AIMP2 in cells, mice reduced α-synuclein aggregation.
Most commentators praised the in vitro findings but questioned whether AIMP2 was driving synucleinopathy in vivo. “The work clearly shows that AIMP2 associates with α-synuclein aggregates and seems to play a role in regulating aggregation,” Hilal Lashuel at the Swiss Federal Institute of Technology, Lausanne, Switzerland, told Alzforum. “However, there is not compelling evidence to support that AIMP2 is indeed what drives α-synuclein aggregation; instead, the data suggest that AIMP2 binds to α-synuclein aggregates after they form.” Mark Cookson at the National Institute on Aging, Baltimore, agrees. Noting that AIMP2 is but one of hundreds of known parkin substrates, he suspects that AIMP2 contributes to α-synuclein aggregation rather than directly causing it.
AIMP2 stands for aminoacyl transfer RNA synthetase complex–interacting multifunctional protein-2. It supports protein synthesis. Previously, Lee had linked AIMP2 to a specific form of dopaminergic neuronal death called parthanatos (Aug 2013 news on Lee et al., 2013). Other researchers had spotted AIMP2 in Lewy bodies in Parkinson’s disease tissue but did not know why it was there (Corti et al., 2003; Ko et al., 2005). Lee wondered if AIMP2 accumulations might spur Lewy body aggregation and, if so, how.
First author Sangwoo Ham and colleagues started by studying if AIMP2 formed its own aggregates. Using thioflavin T, they detected AIMP2 β-sheet amyloid structures in vitro. To see how these might affect the formation of Lewy bodies, they added AIMP2 to α-synuclein aggregation assays, and found it sped up the process twofold. To their eyes, the α-synuclein aggregates looked like those found in Lewy bodies. Incubating both proteins together produced highly dense sheet-like structures, whereas α-synuclein alone formed needle-like aggregates (see image below).
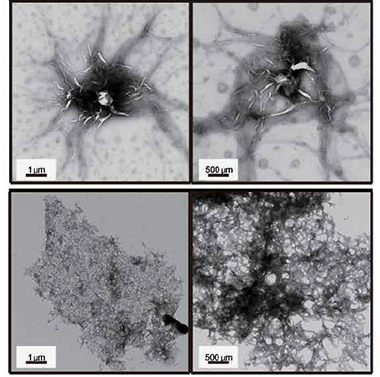
Like a Lewy Body? Aggregates of α-synuclein after incubation with (bottom) or without (top) AIMP2 for seven days. [Courtesy of Ham et al., Science Translational Medicine, 2020.]
Could the same happen in the mouse brain? To find out, the researchers overexpressed both AIMP2 and α-synuclein in dopamine neurons by injecting recombinant adeno-associated viral (rAAV) vectors into the substantia nigra of 2-month-old, wild-type mice. Four weeks later, they found more aggregates in mice overexpressing both AIMP2 and α-synuclein than in mice overexpressing only AIMP2. Eighty percent of the aggregates contained both proteins. The mice overexpressing only α-synuclein showed no aggregation.
Lashuel doubted that the protein inclusions were true aggregates, noting the authors did not characterize them beyond puncta staining. “The paper does not provide convincing evidence for AIMP2’s ability to form amyloid-like oligomers or aggregates in cells or the brain,” he said.
Did these puncta affect neuron function? AIMP2 overexpression alone reduced the number of dopamine neurons by 30 percent, whereas α-synuclein overexpression had no effect. When the researchers overexpressed both proteins, 60 percent of neurons died.
What about mouse behavior? In an open-field test, the mice overexpressing AIMP2 hesitated to explore, which is usually taken as a sign of anxiety. Mice overexpressing both AIMP2 and α-synuclein were even more timid. They also quickly fell off a rotarod and were unable to spread their hindlimbs when picked up by the tail.
All told, the data suggested to the authors that AIMP2 accelerated α-synuclein aggregation, dopaminergic neuron death, and motor function loss. The researchers had seen similar effects in their previous mouse model of AIMP2 overexpression throughout the brain.
What would knocking down or knocking out AIMP2 do to cells and mice? In cultured mouse dopamine neurons, knocking down its expression with shRNA reduced α-synuclein aggregates that otherwise formed after preformed protein fibrils had been introduced into the cells. In wild-type mice, knocking down AIMP2 partially rescued neuron loss otherwise seen after α-synuclein overexpression and fibril seeding; only 45 percent of dopaminergic neurons died, compared with more than 70 percent.

AIMP-ing Up Protection. The neurotoxin 6-OHDA killed about half the dopamine neurons in the substantia nigra of wild-type mice (bottom left), but not of heterozygous AIMP2 knockout mice (bottom right). [Courtesy of Ham et al., Science Translational Medicine, 2020.]
In AIMP2 knockouts, the scientists saw something similar. Homozygous knockout was lethal, but heterozygote pups had no obvious defects, despite having less than half the normal AIMP2 protein levels, they report. This prevented α-synuclein aggregation and neuron loss caused by injecting 6-hydroxydopamine (6-OHDA) into the striatum of 2-month-old knockouts, a commonly used model of parkinsonian damage (Alves da Costa et al., 2006).
Cookson found the AIMP2 knockdown and knockout studies compelling, but was unpersuaded by the overexpression studies. He questioned the inference that AIMP2 aggregation shown in vitro was driving toxicity in the mouse models, bringing up the protein’s proapoptotic effects. “This paper does not resolve whether AIMP2 is triggering cell death, or if neuron death really is through aggregation,” Cookson told Alzforum.
Other commentators, too, appreciated that multiple mouse models showed congruent results in this study. At the same time, they noted discrepancies between this data and previous data from similar models. “They found no α-synuclein pathology in the overexpression model of just α-synuclein, whereas most groups using similar models found robust pathology,” said Michael Henderson from the Van Andel Institute, Grand Rapids, Michigan (see full comment below).
Going back to parkin, what might AIMP2 and α-synuclein interactions mean in PD? In a previously created parkin knockout mouse, there were more aggregates of both AIMP2 and α-synuclein two months after parkin was deleted from the substantia nigra.
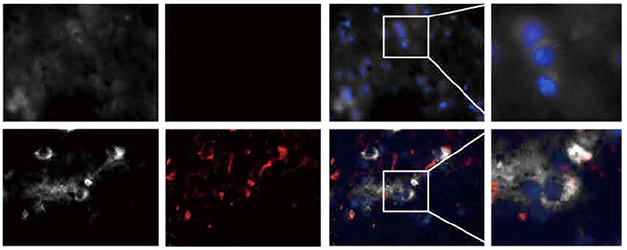
Protein Congregation. AIMP2 (white) and α-synuclein (red) co-localize in Parkinson’s temporal lobe tissue (lower panels), but not in healthy brain (upper panels). [Courtesy of Ham et al., Science Translational Medicine, 2020.]
Whether these findings are relevant to the human brain remains uncertain. Substantia nigra tissue from people who had had Parkinson’s disease with confirmed Lewy bodies did contain more AIMP2 aggregates than control brains, the scientists report. The Lewy bodies contained AIMP2 along with α-synuclein (see image above), but AIMP2 levels varied in the PD samples and the relationship between AIMP2 and α-synuclein aggregation remains unclear.
“Given the toxicity level seen in AIMP2 knockout mice, how druggable is the pathway, and is it actually important to α-synuclein inclusion formation?” Henderson asked.—Chelsea Weidman Burke
References
News Citations
Paper Citations
- Lee Y, Karuppagounder SS, Shin JH, Lee YI, Ko HS, Swing D, Jiang H, Kang SU, Lee BD, Kang HC, Kim D, Tessarollo L, Dawson VL, Dawson TM. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat Neurosci. 2013 Aug 25; PubMed.
- Corti O, Hampe C, Koutnikova H, Darios F, Jacquier S, Prigent A, Robinson JC, Pradier L, Ruberg M, Mirande M, Hirsch E, Rooney T, Fournier A, Brice A. The p38 subunit of the aminoacyl-tRNA synthetase complex is a Parkin substrate: linking protein biosynthesis and neurodegeneration. Hum Mol Genet. 2003 Jun 15;12(12):1427-37. PubMed.
- Ko HS, von Coelln R, Sriram SR, Kim SW, Chung KK, Pletnikova O, Troncoso J, Johnson B, Saffary R, Goh EL, Song H, Park BJ, Kim MJ, Kim S, Dawson VL, Dawson TM. Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J Neurosci. 2005 Aug 31;25(35):7968-78. PubMed.
- Alves DA Costa C, Dunys J, Brau F, Wilk S, Cappai R, Checler F. 6-Hydroxydopamine but not 1-methyl-4-phenylpyridinium abolishes alpha-synuclein anti-apoptotic phenotype by inhibiting its proteasomal degradation and by promoting its aggregation. J Biol Chem. 2006 Apr 7;281(14):9824-31. PubMed.
Further Reading
Papers
- Yun SP, Kim H, Ham S, Kwon SH, Lee GH, Shin JH, Lee SH, Ko HS, Lee Y. VPS35 regulates parkin substrate AIMP2 toxicity by facilitating lysosomal clearance of AIMP2. Cell Death Dis. 2017 Apr 6;8(4):e2741. PubMed.
- Jo A, Lee Y, Park CH, Shin JH. Deubiquitinase USP29 Governs MYBBP1A in the Brains of Parkinson's Disease Patients. J Clin Med. 2019 Dec 24;9(1) PubMed.
- Kim H, Kang SJ, Jo YM, Kim MS, Lee Y, Cho SH, Kim HT. Quantitative analysis of nasal transcripts reveals potential biomarkers for Parkinson's disease. Sci Rep. 2019 Jul 31;9(1):11111. PubMed.
Primary Papers
- Ham S, Yun SP, Kim H, Kim D, Seo BA, Kim H, Shin JY, Dar MA, Lee GH, Lee YI, Kim D, Kim S, Kweon HS, Shin JH, Ko HS, Lee Y. Amyloid-like oligomerization of AIMP2 contributes to α-synuclein interaction and Lewy-like inclusion. Sci Transl Med. 2020 Nov 11;12(569) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Van Andel Institute
While mutations in parkin are associated with early onset parkinsonism, this has generally been thought to be pathologically distinct from idiopathic Parkinson's disease. Most parkin patients who have come to autopsy do not have the α-synuclein Lewy bodies which are diagnostic of Parkinson's disease.
Previous work has demonstrated that parkin knockout in mice does not lead to α-synuclein aggregation either, consistent with human neuropathology. However, these mice do have elevated AIMP2, and previous work has suggested that reduced parkin leads to elevated AIMP2, and increased cell death through parthanatos.
The current paper suggests that, in addition to this putatively α-synuclein-independent mode of cell death leading to parkinsonism, AIMP2 can cause or exacerbate α-synuclein aggregation and may be involved in idiopathic PD. This intriguing finding brings together two distinct fields and could have implications for our understanding of disease pathogenesis.…More
Make a Comment
To make a comment you must login or register.