Could a Failed Heart Drug Treat Tauopathies?
Quick Links
In Alzheimer’s disease and other tauopathies, tau becomes hyperphosphorylated, breaks away from microtubules, and wanders into the cell bodies and dendrites as it begins to aggregate. Which of these steps make the protein toxic? In the September 26 Proceedings of the National Academy of Sciences, scientists led by Eva-Maria and Eckhard Mandelkow of the German Center for Neurodegenerative Diseases (DZNE) in Bonn once again pinpoint aggregation as the culprit.
In a mouse line expressing a form of tau that lacks lysine 280 (ΔK280) and is prone to aggregate, the researchers saw falling ATP levels, faulty synapses, and dampened neuronal activity. These measures were normal in mice expressing ΔK280-PP tau, whose two additional prolines keep the protein from aggregating. Rolofylline, an adenosine receptor antagonist, restored synaptic activity and even corrected behavioral deficits in the ΔK280 mice. The findings suggest that aggregation brings about tau toxicity, and point to rolofylline as a potential drug candidate—at least for some tauopathies.
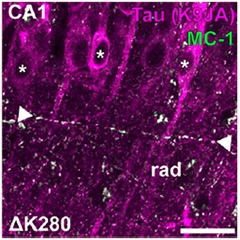
Grainy Image.
The MC-1 antibody (green) detects spindle-shaped grains (white arrows) of tau (purple) in axons that carry the ΔK280 mutation. The grains are absent from the somatodendritic compartment of neurons (asterisks). [Image courtesy of Dennissen, PNAS.]
Deleting lysine at position 280 disrupts tau’s normal interaction with microtubules and renders it prone to aggregate (Barghorn et al., 2000; van Swieten et al., 2007). Scientists found the ΔK280 mutation in a Dutch patient with familial frontotemporal dementia and later in a patient with late-onset AD (Rizzu et al., 1999; Momeni et al., 2009).
While genetic evidence that this mutation causes disease remains weak, ΔK280 transgenic mice do accumulate spindle-like aggregates of tau in their axons. These spindles pack more loosely than neurofibrillary tangles, suggesting they are structurally different. Nevertheless, spindle-shaped grains have been seen in several mouse models of tau pathology and in people who have tau mutations that make the protein more likely to aggregate (Spina et al., 2008; Harris et al., 2012). Although ΔK280 mice develop no neuronal loss, they have reduced long-term potentiation, faulty synaptic plasticity, and severe memory deficits by about 12 months. To see if tau aggregation lay at the root of these problems, the Mandelkow group made a similar ΔK280 model with two extra prolines in tau that break up β-sheets (ΔK280-PP). This protein has the same affinity for microtubules as ΔK280 tau, but does not aggregate. ΔK280-PP mice have no memory impairments, though it is unclear why (van der Jeugd et al., 2012).
To find out what distinguishes ΔK280 mice from the ΔK280-PP strain, Dennissen examined hippocampal slices from both animals. Both types of tau were phosphorylated on the same amino acids and both missorted into the cell bodies and dendrites. However, while the ΔK280 neurons had fewer axonal mitochondria, less ATP, and fewer dendritic spines, the ΔK280-PP cells appeared normal (see image below). Further, only ΔK280tau formed grains in the axons as detected by MC-1, an antibody that detects a conformation of tau that has been associated with pathology (see image above). All told, the data indicated to the authors that aggregation, rather than missorting or hyperphosphorylation, made tau toxic. Dennissen also noted a defect in short-term synaptic plasticity and depressed basal synaptic transmission in the ΔK280 mice only.
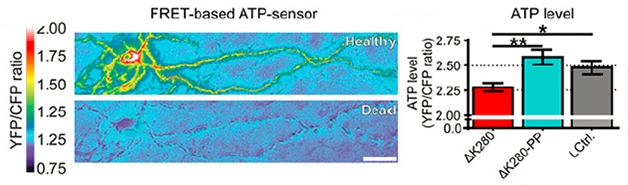
Low energy: A healthy neuron (left; gray bar on right) produces robust ATP, even if it expresses ΔK280-PP tau (turquoise bar). Cells expressing ΔK280 tau make less ATP (red bar). [Image courtesy of Dennissen, PNAS.]
Dennissen went hunting for clues to these changes. He first used quantitative PCR to look for genes that were up- or downregulated. Both cFOS and GFAP were below normal, suggesting floundering neuronal and astrocytic activity, respectively. To their surprise, the authors detected no changes in expression of protein degradation genes, or genes that would hint at osmotic, oxidative, or other types of molecular stress. So how was ΔK280 tau suppressing neuronal activity?
In sifting through the literature for an answer, Dennissen found that activating the adenosine A1 receptor wrought a similar type of havoc as expressing ΔK280 tau: reduced long-term potentiation, impaired synapses, faltering neural activity (for a review, see Dias et al., 2015). He wondered if antagonizing that receptor would counteract the effects of aggregated tau.
To find out, he treated hippocampal slices from ΔK280 mice with rolofylline, an experimental A1 receptor antagonist that had recently failed in a Phase 3 trial for cardiac failure (Massie et al., 2010). In the mice, the compound normalized dendritic spine number, presynaptic function, and neuronal activity. In 14-month-old ΔK280 mice, two weeks of rolofylline restored their preference for exploring the unfamiliar arm of a Y-maze or a novel object, as well as their ability to associate an auditory cue with a foot shock. This series of tests suggested that spatial memory, long-term object recognition, and contextual memory were restored. “That was the main surprise,” said Eva-Maria Mandelkow, “These mice were completely demented, but all of a sudden they could learn again.” After 10 weeks of treatment, basal synaptic transmission returned to normal levels in hippocampal slices from treated ΔK280 mice.
The authors are unsure how tau reduces neuronal activity, but have a hunch it has to do with adenosine. They point out that ΔK280 neurons have less ATP, which could mean ATP is released from the cell. This would raise extracellular adenosine, which inhibits neuronal activity when it binds to A1 receptors. This may explain why aggregated tau reduced neuronal activity, and why blocking the adenosine A1 receptor can reverse the effects, the authors wrote.
While the drug provided no benefit to cardiac patients, it had few side effects. Might it be a candidate for tauopathies? Mandelkow said that if additional positive data comes in from other mouse, cell, and worm models, her group will seek to collaborate on a Phase 1 trial in a small number of AD patients to see if rolofylline is safe and improves network activity. The group is currently testing rolofylline in mouse models carrying P301L tau. Abid Hussaini, Columbia University Medical Center, New York, cautioned that boosting neuronal activity might be deleterious in tauopathies. Hussaini and colleagues recently reported that activity can accelerate the spread of tau to adjacent neurons (Jun 2016 news). “It is important to identify the underlying mechanism that causes the dysfunction before attempting to correct it,” he said.
Eckhard Mandelkow pointed out that the adenosine A1 receptor on neurons differs from the A2A adenosine receptor that sits on astrocytes. Lennart Mucke’s group at the Gladstone Institute of Neurological Disease, San Francisco, recently reported that knocking out this astrocyte receptor improves memory in AD mouse models (Jan 2015 news; Dec 2014 conference news). Given that finding, Adam Boxer, University of California, San Francisco, wondered whether rolofylline could be antagonizing astrocytic A2A receptors as well. Dennissen noted that rolofylline is 890 times more selective for A1 versus A2A (Nonaka et al., 1996).
“We don't have a huge pipeline for tau-directed therapies, and it would be worthwhile to further pursue novel approaches such as this one,” Boxer wrote to Alzforum. “However, [rolofylline] might be more of a symptomatic agent than disease-modifying, which might reduce enthusiasm for clinical development,” he added.—Gwyneth Dickey Zakaib
References
Research Models Citations
Antibody Citations
News Citations
- Excited Neurons Release More Aberrant Tau
- Paper Alert: Astrocyte Receptor Could Hinder Memory
- Do Astrocyte Receptors Go Over the Top in Alzheimer’s?
Paper Citations
- Barghorn S, Zheng-Fischhöfer Q, Ackmann M, Biernat J, von Bergen M, Mandelkow EM, Mandelkow E. Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry. 2000 Sep 26;39(38):11714-21. PubMed.
- van Swieten JC, Bronner IF, Azmani A, Severijnen LA, Kamphorst W, Ravid R, Rizzu P, Willemsen R, Heutink P. The DeltaK280 mutation in MAP tau favors exon 10 skipping in vivo. J Neuropathol Exp Neurol. 2007 Jan;66(1):17-25. PubMed.
- Rizzu P, Van Swieten JC, Joosse M, Hasegawa M, Stevens M, Tibben A, Niermeijer MF, Hillebrand M, Ravid R, Oostra BA, Goedert M, van Duijn CM, Heutink P. High prevalence of mutations in the microtubule-associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am J Hum Genet. 1999 Feb;64(2):414-21. PubMed.
- Momeni P, Pittman A, Lashley T, Vandrovcova J, Malzer E, Luk C, Hulette C, Lees A, Revesz T, Hardy J, de Silva R. Clinical and pathological features of an Alzheimer's disease patient with the MAPT Delta K280 mutation. Neurobiol Aging. 2009 Mar;30(3):388-93. Epub 2007 Aug 27 PubMed.
- Spina S, Farlow MR, Unverzagt FW, Kareken DA, Murrell JR, Fraser G, Epperson F, Crowther RA, Spillantini MG, Goedert M, Ghetti B. The tauopathy associated with mutation +3 in intron 10 of Tau: characterization of the MSTD family. Brain. 2008 Jan;131(Pt 1):72-89. Epub 2007 Dec 7 PubMed.
- Harris JA, Koyama A, Maeda S, Ho K, Devidze N, Dubal DB, Yu GQ, Masliah E, Mucke L. Human P301L-mutant tau expression in mouse entorhinal-hippocampal network causes tau aggregation and presynaptic pathology but no cognitive deficits. PLoS One. 2012;7(9):e45881. PubMed.
- Van der Jeugd A, Hochgräfe K, Ahmed T, Decker JM, Sydow A, Hofmann A, Wu D, Messing L, Balschun D, D'Hooge R, Mandelkow EM. Cognitive defects are reversible in inducible mice expressing pro-aggregant full-length human Tau. Acta Neuropathol. 2012 Jun;123(6):787-805. PubMed.
- Dias RB, Rombo DM, Ribeiro JA, Henley JM, Sebastião AM. Adenosine: setting the stage for plasticity. Trends Neurosci. 2013 Apr;36(4):248-57. Epub 2013 Jan 15 PubMed.
- Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC, PROTECT Investigators and Committees. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010 Oct 7;363(15):1419-28. PubMed.
- Nonaka H, Ichimura M, Takeda M, Kanda T, Shimada J, Suzuki F, Kase H. KW-3902, a selective high affinity antagonist for adenosine A1 receptors. Br J Pharmacol. 1996 Apr;117(8):1645-52. PubMed.
Further Reading
Papers
- Pir GJ, Choudhary B, Mandelkow E, Mandelkow EM. Tau mutant A152T, a risk factor for FTD/PSP, induces neuronal dysfunction and reduced lifespan independently of aggregation in a C. elegans Tauopathy model. Mol Neurodegener. 2016 Apr 27;11:33. PubMed.
- Silva MC, Cheng C, Mair W, Almeida S, Fong H, Biswas MH, Zhang Z, Huang Y, Temple S, Coppola G, Geschwind DH, Karydas A, Miller BL, Kosik KS, Gao FB, Steen JA, Haggarty SJ. Human iPSC-Derived Neuronal Model of Tau-A152T Frontotemporal Dementia Reveals Tau-Mediated Mechanisms of Neuronal Vulnerability. Stem Cell Reports. 2016 Sep 13;7(3):325-340. Epub 2016 Sep 1 PubMed.
- Gerson J, Kayed R. Therapeutic Approaches Targeting Pathological Tau Aggregates. Curr Pharm Des. 2016;22(26):4028-39. PubMed.
- Van der Jeugd A, Hochgräfe K, Ahmed T, Decker JM, Sydow A, Hofmann A, Wu D, Messing L, Balschun D, D'Hooge R, Mandelkow EM. Cognitive defects are reversible in inducible mice expressing pro-aggregant full-length human Tau. Acta Neuropathol. 2012 Jun;123(6):787-805. PubMed.
Primary Papers
- Dennissen FJ, Anglada-Huguet M, Sydow A, Mandelkow E, Mandelkow EM. Adenosine A1 receptor antagonist rolofylline alleviates axonopathy caused by human Tau ΔK280. Proc Natl Acad Sci U S A. 2016 Oct 11;113(41):11597-11602. Epub 2016 Sep 26 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.