In Colombian Alzheimer’s Kindred, Blood NfL Climbs 22 Years Before Symptoms
Quick Links
In carriers of a pathogenic mutation in presenilin 1, plasma levels of the neurodegeneration marker neurofilament light (NfL) start to diverge from those in noncarriers more than two decades prior to when Alzheimer’s symptoms begin. That was the upshot of a study published May 26 in the Lancet Neurology. Led by Kaj Blennow at the University of Gothenberg in Sweden and Francisco Lopera of Universidad de Antioquia in Medellin, Colombia, the study leveraged both longitudinal and cross-sectional data on blood samples that more than 2,000 members of the Colombian kindred harboring the PSEN1-E280A mutation had donated to help scientists chart the rise of biomarkers of AD.
- Massive study measured plasma NfL in Colombian familial AD kindred.
- As a group, carriers diverged from noncarriers 22 years before estimated symptom onset.
- In individuals, the marker distinguished carriers and noncarriers three years before onset.
“In general, this and other data in studies of neurodegenerative diseases suggest that serum/plasma NfL is a marker of neurodegeneration that will be useful in prognosis as well as in assessing response to disease-modifying therapies,” wrote David Holtzman of Washington University in St. Louis in a comment to Alzforum.
Alzforum covered the findings when Eric Reiman of Banner Alzheimer’s Institute in Phoenix presented them at AAIC last year (Aug 2019 conference news).
The study included 1,070 mutation carriers and 1,074 age-matched noncarriers with baseline data, as well as 242 carriers and 262 noncarriers with serial measurements. Participants ranged from 8 to 75 years of age. Gothenberg scientists Blennow and Henrik Zetterberg used an ultrasensitive Simoa assay to measure plasma NfL.
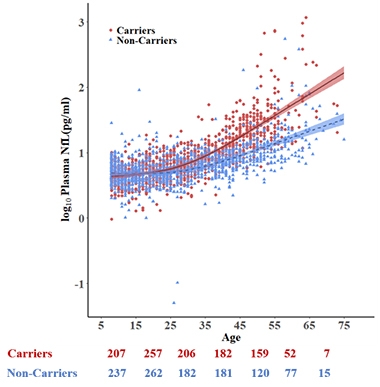
Rise of the NfL. Plasma NfL rises with age in both carriers (red) and noncarriers (blue). By age 22, carriers have higher plasma NfL than do noncarriers. Estimated symptom onset age is 44. [Courtesy of Quiroz et al., Lancet Neurology, 2020.]
Using the cross-sectional data, co-first authors Yakeel Quiroz of Massachusetts General Hospital in Boston, Zetterberg, and Reiman found that while plasma NfL crept up with age in both carriers and noncarriers, the average NfL concentration of carriers split from noncarriers at age 22, which also happens to be 22 years prior to estimated symptom onset. Groups overlapped significantly, especially at younger ages. Not until three years prior to symptom onset were the differences in plasma NfL large enough to sensitively distinguish individual carriers from noncarriers.
In a subset of 504 participants who were followed longitudinally for an average of six years, baseline plasma NfL correlated with its subsequent rise in carriers. Baseline NfL also predicted subsequent slippage on the MMSE in both carriers and noncarriers, and in memory scores in carriers.
The findings mesh with those recently reported from the Dominantly Inherited Alzheimer’s Network, in which plasma NfL diverged in mutation carriers starting 16 years prior to symptom onset (Jan 2019 news). Holtzman chalked up the earlier detection of rising plasma NfL in the Colombian cohort to its larger size, and to the fact that its members all carry the same mutation. The DIAN cohort comprises more than 250 different pathogenic mutations in APP, presenilin 1, and presenilin 2.
Mathias Jucker of the German Center for Neurodegenerative Diseases, Tübingen, was pleased to see convergence between two separate autosomal-dominant AD data sets. “It is my understanding that these data also match with the more difficult-to-do studies in sporadic AD. It confirms that NfL is an important readout when we aim at presymptomatic treatment,” Jucker wrote to Alzforum.
Leslie Shaw of the University of Pennsylvania in Philadelphia noted that NfL is a general marker of degenerating neurons, whose plasma concentration can rise due to all sorts of non-AD pathologies or other neuronal insults. Still, it may serve as a useful gauge of disease stage when combined with specific markers of amyloid and tau pathology. “Future studies are ongoing and needed to define how well plasma NfL reflects neurodegeneration and adds value in prediction of disease state and cognitive decline in the individual patient,” Shaw wrote. —Jessica Shugart
References
Mutations Citations
News Citations
Further Reading
No Available Further Reading
Primary Papers
- Quiroz YT, Zetterberg H, Reiman EM, Chen Y, Su Y, Fox-Fuller JT, Garcia G, Villegas A, Sepulveda-Falla D, Villada M, Arboleda-Velasquez JF, Guzmán-Vélez E, Vila-Castelar C, Gordon BA, Schultz SA, Protas HD, Ghisays V, Giraldo M, Tirado V, Baena A, Munoz C, Rios-Romenets S, Tariot PN, Blennow K, Lopera F. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer's disease kindred: a cross-sectional and longitudinal cohort study. Lancet Neurol. 2020 Jun;19(6):513-521. Epub 2020 May 26 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Pennsylvania Medical Center
This is a very clearly and thoughtfully described study of plasma NfL in the Colombian family with the presenilin-1 E280A mutation. Given the large age range (8 to 75) of this cohort and large number (>2,000) of enrolled mutation carriers (impaired and unimpaired) and age-matched noncarriers of the mutation, and inclusion of more than 400 sets of individual-subject longitudinal data, the study permitted a robust analysis of cross-sectional and longitudinal plasma NfL data with key findings. They are:
Interpretation at the older ages is clouded by the overall increases with age of NfL plasma concentrations. Thus at a group level, the increased plasma concentrations of NfL in mutation carriers relative to noncarriers supports the hypothesis that this biomarker shows increased neurodegeneration over time. However, the authors point out it is not a robust test for disease presence or absence in the individual patient.
This study data contributes in a number of ways to the study data provided by the DIAN study, including the above group-level increases over time in mutation carriers that are higher than in noncarriers and provides additional data by extending the timeline of changes in this genetically defined AD population to that provided by DIAN (Preische et al., 2019) and other ADAD studies, such as that of Weston et al. (2019).
Among the added values of this study is its clear and strong support for, and interest in, describing the need for further studies involving NfL in this and other study populations that will include combinations of biofluid and imaging biomarkers in order to assess what are the most useful combinations of biomarkers for disease detection over the continuum of Alzheimer’s disease. That will help to improve detection of disease stage and risk for decline in individual patients.
The overall wide range of NfL plasma concentrations observed at various times across the wide study timeline can reflect influences of co-pathologic processes such as Lewy body pathology, known to be highly prevalent in ADAD and in sporadic AD. It could also reflect vascular changes such as cerebral amyloid angiography, also known to be prevalent in genetic and sporadic forms of the disease (Toledo et al., 2013; Cairns et al., 2015; Ringman et al., 2016). Other possibilities include the presence or absence of genetic markers such as APOE ε4, and other factors including pre-analytic steps as well as analytical methodology.
NfL is described in this and other studies as a biomarker of neurodegeneration. This process is not specific for this disease, but is potentially highly valuable in defining this aspect of disease pathology. When combined with a measure of amyloid and of tau pathology, NfL can improve upon assessment of disease stage and predictive performance for future cognitive decline. Future studies are ongoing and needed to define how well plasma NfL reflects neurodegeneration and adds value in prediction of disease state and cognitive decline in the individual patient.
References:
Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C, Gräber S, Kuder-Buletta E, LaFougere C, Laske C, Vöglein J, Levin J, Masters CL, Martins R, Schofield PR, Rossor MN, Graff-Radford NR, Salloway S, Ghetti B, Ringman JM, Noble JM, Chhatwal J, Goate AM, Benzinger TL, Morris JC, Bateman RJ, Wang G, Fagan AM, McDade EM, Gordon BA, Jucker M, Dominantly Inherited Alzheimer Network. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019 Feb;25(2):277-283. Epub 2019 Jan 21 PubMed.
Weston PS, Poole T, O'Connor A, Heslegrave A, Ryan NS, Liang Y, Druyeh R, Mead S, Blennow K, Schott JM, Frost C, Zetterberg H, Fox NC. Longitudinal measurement of serum neurofilament light in presymptomatic familial Alzheimer's disease. Alzheimers Res Ther. 2019 Feb 20;11(1):19. PubMed.
Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, Householder E, Ayutyanont N, Roontiva A, Bauer RJ, Eisen P, Shaw LM, Davatzikos C, Weiner MW, Reiman EM, Morris JC, Trojanowski JQ, Alzheimer’s Disease Neuroimaging Initiative (ADNI). Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun. 2013 Oct 9;1(1):65. PubMed.
Cairns NJ, Perrin RJ, Franklin EE, Carter D, Vincent B, Xie M, Bateman RJ, Benzinger T, Friedrichsen K, Brooks WS, Halliday GM, McLean C, Ghetti B, Morris JC, Alzheimer Disease Neuroimaging Initiative, Dominantly Inherited Alzheimer Network. Neuropathologic assessment of participants in two multi-center longitudinal observational studies: the Alzheimer Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN). Neuropathology. 2015 Aug;35(4):390-400. Epub 2015 May 12 PubMed.
Ringman JM, Monsell S, Ng DW, Zhou Y, Nguyen A, Coppola G, Van Berlo V, Mendez MF, Tung S, Weintraub S, Mesulam MM, Bigio EH, Gitelman DR, Fisher-Hubbard AO, Albin RL, Vinters HV. Neuropathology of Autosomal Dominant Alzheimer Disease in the National Alzheimer Coordinating Center Database. J Neuropathol Exp Neurol. 2016 Mar;75(3):284-90. Epub 2016 Feb 17 PubMed.
Washington University
This paper shows that, in a very large number of presenilin-1 E280A mutation carriers (Columbian kindred) and age-matched controls, plasma NfL is higher. Statistically, cross-sectional values begin to diverge ~22 years before expected age of onset but assessing these values does not differentiate mutations carriers vs. noncarriers reliably until ~ three years prior to expected age of onset.
These data are very similar to those reported from the dominantly inherited Alzheimer’s network - DIAN (Preische et al., 2019). The main differences between the 2 studies were:
In general, this and other data in studies of neurodegenerative diseases suggest that serum/plasma NfL is a marker of neurodegeneration that will be useful in prognosis as well as in assessing response to disease modifying therapies.
References:
Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C, Gräber S, Kuder-Buletta E, LaFougere C, Laske C, Vöglein J, Levin J, Masters CL, Martins R, Schofield PR, Rossor MN, Graff-Radford NR, Salloway S, Ghetti B, Ringman JM, Noble JM, Chhatwal J, Goate AM, Benzinger TL, Morris JC, Bateman RJ, Wang G, Fagan AM, McDade EM, Gordon BA, Jucker M, Dominantly Inherited Alzheimer Network. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019 Feb;25(2):277-283. Epub 2019 Jan 21 PubMed.
Make a Comment
To make a comment you must login or register.