Cerebrospinal Fluid Neurogranin Correlates with Markers of Neurodegeneration
Quick Links
Since they first observed neurogranin in the cerebrospinal fluid (CSF) of Alzheimer’s disease patients, researchers have suspected that the synaptic protein may be a useful surrogate for measuring synapse loss in the brain. Now, a paper published September 15 in Brain reports that CSF neurogranin correlates with glucose hypometabolism and hippocampal atrophy, markers of neurodegeneration. The findings follow hot on the heels of a report linking CSF neurogranin to impending cognitive decline (see Sept 2015 news).
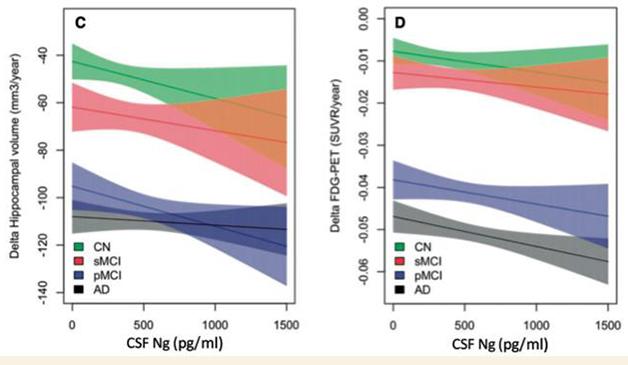
Markers of Neurodegeneration? Baseline neurogranin levels associate with hippocampal atrophy and glucose hypometabolism, two measures of neurodegeneration. [Courtesy of Erik Portelius and Brain. © Oxford University Press.]
Erik Portelius and colleagues at University of Gothenburg, Sweden, used their in-house immunoassay to measure CSF neurogranin levels in a well-characterized cohort from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). It included 95 people with AD, 173 with mild cognitive impairment, and 110 cognitively healthy controls. Participants were followed clinically for six months or more, and were repeatedly scanned by FDG-PET and MRI over a period of six months to nine years.
Neurogranin levels were not associated with baseline hippocampal volume or FDG-PET in any group. However, high baseline CSF neurogranin did correlate with future loss of hippocampal volume and reduction in cortical glucose metabolism in MCI patients. Neurogranin levels also correlated with cognitive decline in this group.
“In CSF neurogranin, we now have an independent marker of synaptic integrity, which previously has been very difficult to assess in living patients,” said Portelius. He added that the link between neurogranin and brain imaging measures needs to be replicated in other cohorts, particularly in large preclinical populations.
Maartje Kester, VU University Medical Center, Amsterdam, who was not involved in this study, noted that its neurogranin results align with those reported by her group earlier this week (Kester et al., 2015). Studying the Amsterdam Dementia Cohort, they found neurogranin levels to be elevated by the MCI stage of AD, possibly earlier, as suggested by the longitudinal increase in CSF neurogranin among those with subjective memory complaint. “Taken together, these results confirm the value of neurogranin as a promising early biomarker reflecting functional decline,” Kester wrote to Alzforum.
Consistent results observed in different cohorts using different assays are encouraging, but even so, questions remain before neurogranin is promoted from “promising biomarker” to being useful for diagnosis and/or prognosis, said co-author Kaj Blennow, also from Gothenburg. “We now need to understand the mechanism behind the increase of CSF neurogranin in AD, and whether it is specific for AD or also occurs in other neurodegenerative disorders that have synapse loss,” Blennow said.—Kelly Dakin
References
News Citations
Paper Citations
- Kester MI, Teunissen CE, Crimmins DL, Herries EM, Ladenson JH, Scheltens P, van der Flier WM, Morris JC, Holtzman DM, Fagan AM. Neurogranin as a Cerebrospinal Fluid Biomarker for Synaptic Loss in Symptomatic Alzheimer Disease. JAMA Neurol. 2015 Nov;72(11):1275-80. PubMed.
Further Reading
Primary Papers
- Portelius E, Zetterberg H, Skillbäck T, Törnqvist U, Andreasson U, Trojanowski JQ, Weiner MW, Shaw LM, Mattsson N, Blennow K, Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer's disease. Brain. 2015 Nov;138(Pt 11):3373-85. Epub 2015 Sep 15 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
The findings of Portelius and colleagues are in line with our recent results (Kester et al,. 2015) and confirm the value of neurogranin as a CSF biomarker for AD. Both studies show the predictive value of neurogranin for disease progression in the MCI phase of AD. In addition, Portelius and colleagues report that baseline levels of neurogranin associate with longitudinal imaging biomarkers, MRI and PET. These associations strengthen the hypothesis that CSF neurogranin is an early biomarker for AD that reflects synaptic degeneration. Further studies are needed to confirm the association between neurogranin and these, and other, biomarkers.
Eisai Inc.
Miguel de Cervantes European University (UEMC)
Neurogranin is a protein found at high concentrations in the specialized regions of nerve cells that enable them to receive synaptic signals (the postsynaptic terminal). The function of neurogranin is not yet well understood, but it is known to bind to another protein called calmodulin, which mediates some forms of synaptic plasticity. Levels of neurogranin have been recently found to be increased in the cerebrospinal fluid (CSF) of patients with Alzheimer's disease (AD), potentially serving as an early biomarker of synaptic damage and/or pathology.
This new study by Portelius and colleagues confirmed the presence of higher CSF neurogranin levels in both AD and MCI subjects compared with heathy controls. More importantly, the authors demonstrated that CSF neurogranin could distinguish MCI subjects who progressed to dementia from those who remained stable. Additionally, high baseline CSF neurogranin levels predicted longitudinal cognitive decline, longitudinal reductions in cortical glucose metabolism and hippocampal volume at clinical follow-up. Taken together, these data suggest that neurogranin in the CSF of individuals newly diagnosed with MCI may inform physicians about the patient’s risk of future cognitive decline and structural/functional brain changes.
The main findings of this study remind us that MCI is a heterogeneous concept. Many subjects with MCI may stay clinically stable for years while others can be subject to a rapid decline. Physicians need a way to identify subjects at the greatest risk of progression as early as possible. The results shown here hold promise that systematic measurement of neurogranin in the CSF will support risk evaluation for metabolic, structural, and cognitive disease progression and monitor response to therapy of MCI subjects in future treatment trials.
However, some issues are slowing the development of robust biomarkers, and the need for standardization remains one of the main challenges. To confirm neurogranin as a reliable marker of disease progression, investigators need large sets of samples that have been all collected in a standardized manner and investigated, multicentric, in independent samples by independent expert research groups. Thus, Portelius and colleagues' use of samples collected in a biorepository managed by an internationally established large-scale consortium such as the Alzheimer’s Disease Neuroimaging Initiative (ADNI) should be clearly appreciated.
In the setting of MCI or prodromal AD, neurogranin may be useful as a biomarker for drugs specifically targeting synaptic dysfunction. Once standardization issues have been definitively overcome, the best proof of concept for neurogranin in MCI would be to show its correlation with cognitive benefits during a successful drug trial. Perhaps neurogranin could be a promising candidate for surrogate marker development.
University of California, San Diego
Neurogranin appears to be an interesting and novel marker that appears to reflect post-synaptic damage. There are multiple proteolytically cleaved forms of neurogranin in CSF; this is reminiscent of tau, with which neurogranin correlates. It is possible that similar proteolytic events release at least some forms of tau that are detected in CSF as well as neurogranin, which would account for the high correlation between the two.
The correlation between baseline neurogranin levels and progressive changes that reflect neuodegeneration, such as MRI atrophy and FDG PET, suggests that the tempo of synaptic damage carries some predictive value for progression of AD.
Further studies should be aimed at identifying how early in the course of AD neurogranin levels change in the CSF. Also, it would be interesting to evaluate levels in acute situations such as stroke and TBI (where CSF tau is increased) and after seizures.
The development of neurogranin as a synaptic damage biomarker adds to the repertoire of biomarkers in AD. It will be interesting to see it routinely applied to clincial trials as an outcome measure.
The recent report of Portelius and colleagues demonstrating patterns of CSF neurogranin in a relatively large ADNI cohort adds important information to what has already been published about this promising biomarker, notably its relationship with structural imaging measures, cortical glucose metabolism and cognitive performance. As has been found with many CSF biomarkers, abnormalities are observed in the MCI stage, prior to the onset of dementia, and track with clinical and pathologic disease progression.
The question, of course, remains as to whether changes are taking place even earlier than MCI, prior to any cognitive symptoms (preclinical AD), and what the relationships are between levels of neurogranin and the other biomarkers within individuals over time.
In terms of potential clinical use, we need to know whether analysis of neurogranin (or other synaptic markers) adds information beyond what CSF tau and phospho-tau already provide. One hypothesis is that changes in synaptic markers will be detectable earlier than tau-related measures of neurodegeneration. However, given that synaptic dysfunction/loss is not unique to AD, it is likely that other markers, such as those for amyloid, will also need to be included in order to establish AD as the underlying pathologic disorder. Longitudinal evaluation of large cohorts of cognitively normal individuals who undergo CSF collection and imaging and cognitive follow-up will be necessary to test this hypothesis; these studies are currently ongoing.
Biomarkable bvba
Co-founder of ADx NeuroSciences and founder of Key4AD
�
Accumulating evidence from studies such as this illustrates that synapse proteins, and neurogranin in particular, might become important biomarkers to estimate the progression of Alzheimer's disease. However, several aspects have to be considered here. As for other biomarkers, neurogranin is present in CSF in many different isoforms. Therefore, full characterization of the nature of neurogranin detected in a biological sample is of utmost importance. In the past, for example, it was reported that assays for specific forms of tau protein can enhance the diagnostic performance of this biomarker (Meredith et al., 2013).
Although Portelius and colleagues have substantially contributed to the research on neurogranin isoforms (Kvartsberg et al., 2014), in this study their assay design did not quantify specific forms of pre-defined neurogranin fragments. This provides an incentive to use more specific assays to define which forms of neurogranin are the most diagnostically relevant. In addition, the assay in the current study employs a polyclonal antibody. For long-term availability and for integration into larger studies or clinical trials, assays need to be developed that are entirely based on monoclonal antibodies. At ADx NeuroSciences in Gent, Belgium, we are currently developing such a commercial product, targeting a specific truncated form of neurogranin that is abundantly present in CSF.
As the next step in this early phase of validating neurogranin as an AD biomarker, we would advise that all the different assay formats currently available in the field be compared, if possible using samples obtained from consortia, larger follow-up studies and/or clinical trials. Open sharing of these data will not only considerably help the community in verifying the biological value of neurogranin. It could also identify the most optimal assay format (critical raw materials being antibodies and calibrators) detecting specific forms of the protein, as a tool for patient stratification, follow-up and treatment monitoring.
In this respect, it is possible that such a verified assay format might be a combination of neurogranin with other biomarkers, i.e. tau, Aβ or other (synaptic) proteins. Several studies do indeed indicate that, for example, the ratio of CSF A β1-42/CSF tau has a higher diagnostic strength than the single analytes. This particular ratio can even identify early AD with an accuracy equal to amyloid PET imaging (Palmqvist et al., 2015; Vanderstichele et al., in preparation). Palmqvist et al nicely demonstrated that you can use either CSF analysis or a PET scan for early diagnosis of subjects with AD pathology.
The addition of neurogranin to the biomarker panel will allow the field to predict cognitive deterioration and disease-associated changes over time, i.e. to have a progression marker. To qualify neurogranin as a new biomarker, we need larger collaborative efforts on longitudinal samples, using harmonized assays (see Bjerke et al., 2015 in press) and fully detailed collection and storage procedures for the biological samples, i.e. defined pre-analytical variables.
Critical Path Institute
Critical Path Institute
There has long been hope for identification of biomarkers that track with synaptic loss and clinically meaningful change. At present there is no regulatory endorsement by the FDA as a prognostic biomarker in AD clinical trials. On behalf of the Critical Path Institute’s Coalition Against Major Diseases (CAMD), we have featured CSF neurogranin as a promising candidate biomarker to the FDA based on the exciting work featured in this new publication. This work in combination with literature by these and other investigators collectively support what we view as “regulatory ready” and pave the way for the future.
CAMD’s long journey aiming to achieve FDA biomarker qualification of CSF analytes Aβ1-42, tau, and p-tau as prognostic biomarkers for enrichment in prodromal AD clinical trials is worth noting. Such issues deserve to gain visibility in order to learn from the past and gain efficiency for consortia-driven initiatives in advancing promising new biomarkers such as neurogranin for the future.
CSF analytes have a rich and impressive literature of independent studies demonstrating their value in terms of defining subjects at the pre-dementia stage more likely to progress to AD dementia. The use of CSF analytes Aβ1-42, tau, and p-tau at baseline in AD clinical trials for the purposes of enrichment of those subjects who have MCI due to AD is commonly used, yet sponsors own the risk of choosing the appropriate assay, reference materials, cutpoint determination strategies, and assuring reliability and reproducibility of the CSF assay platform over long-duration recruitment times.
Access to CSF samples is a key challenge for all stakeholders advancing CSF candidates to regulatory authorities. To date, the lack of sufficient access to data has been an impediment to the field’s ability to support FDA biomarker qualification. In order to prompt further data sharing and attention to standardization, the FDA issued a letter of support encouraging the use of CSF analytes in AD clinical trials.
The costs and risks for independent organizations embarking on regulatory endorsement of biomarkers are daunting. Learnings from the numerous amyloid PET approvals represent notable examples. There is an urgent need to establish successful big-data platforms for sharing clinical biomarker data integrated with clinical and genetic data. We would like to point to the CAMD Alzheimer’s disease database, which was fundamental to developing the first-ever regulatory-endorsed quantitative clinical trial drug development tool.
The recognition of the need for collaboration to develop effective therapeutics in AD is clearly improving, but barriers still exist. Several groups, such as CAMD and the Alzheimer’s Association-funded Global Biomarkers Standardization Consortium (GBSC), have been working together for some time to share resources, learnings, and even data. However, this is not occurring at the level that is needed for true global alliances, particularly in regard to data sharing. We need to incentivize open sharing of biomarker data, including validation data such as test-retest reliability, as well as clinical performance data from stakeholders including manufacturers and diagnostic companies.
Additional barriers to success include the lack of standardized bio-banking and globally accessible bio-repositories. CSF samples from AD clinical trials are extremely valuable, and consent should be broadened so that precious samples and the patients' commitment and sacrifice are not lost.
We also need to incentivize the use of biomarker AD standards such as the AD Clinical Data Interchange Standardization Consortium (CDISC) standards (including CSF biomarkers). Standardization does not stand in contrast to innovation. The FDA’s new mechanism to accelerate the awareness of promising biomarkers by issuing a “letter of support” will hopefully catalyze further recognition of this importance.
Make a Comment
To make a comment you must login or register.