BACE Inhibitors Hitch Ride into Endosomes via Membrane Anchor
Quick Links
Location, location, location. Among those who suggest this real estate mantra deserves higher priority in Alzheimer disease drug design are scientists in Germany who have created a membrane-tethered version of a β-secretase inhibitor. In today’s issue of Science, the researchers report that this modified inhibitor reaches endosomes, the subcellular compartments containing active β-secretase, and greatly outperforms free inhibitor at blocking the enzyme’s activity in cultured cells and in fruit fly and mouse models. While these findings represent a “proof of principle” for the membrane-anchoring approach, the new strategy faces tough hurdles en route to practical drug therapy.
BACE1, the transmembrane protein that confers β-secretase activity, has become a prime AD drug target in large part because it plays a key role in generating Aβ peptide, whose accumulation in plaques represents a hallmark feature of AD. Partnering with a complex of proteins known as γ-secretase, BACE1 catalyzes the rate-limiting step in the sequential cleavage of Aβ from amyloid precursor protein (APP) (Small and Gandy, 2006). Like other membrane proteins, BACE1 transits constantly from the plasma membrane into endosomes and back. But from the AD perspective, the dirty work of BACE1—providing the initiating cut of APP for Aβ release—occurs not at the cell membrane but within early endosomes (see Rajendran et al., 2006; ARF related news story). This discovery—reported by Kai Simons and colleagues at the Max Planck Institute for Molecular Cell Biology and Genetics, Dresden—led the authors to consider that targeting BACE1 inhibitors to endosomes might boost their efficiency at reducing harmful β-secretase activity.
Noting the acidic interior of endosomes (pH 5) and contrasting it with the cell exterior, whose pH is closer to 7, first author Lawrence Rajendran reasoned that BACE1 does not adopt its active conformation at the plasma membrane. “If transition-state inhibitors recognize, bind to, and inactivate only the active conformation of the enzyme,” Rajendran told ARF in an interview, “what is the point of designing inhibitors that cannot go to the subcellular compartment where the enzyme is active?”
To coax a β-secretase inhibitor into endosomes, Rajendran and colleagues started with a non-permeable, transition-state, peptide BACE1 inhibitor and used a polylinker to hook it to a cholesterol molecule. The attached sterol moiety, they reasoned, would anchor the inhibitor to the plasma membrane, from which it could hitch a ride on the endocytic trafficking system to reach its target—active β-secretase. Targeting the endocytic pathway would also seem wise in light of recent work showing that 70 percent of Aβ production in the brain requires endocytosis (see ARF related news story).
The membrane-anchoring strategy worked beautifully. In electrochemiluminescence and immunoblotting experiments, the researchers found that treating cultured cells with the sterol-linked inhibitor reduced levels of β-secretase cleavage product in the culture medium by about 80 percent, whereas free inhibitor had no effect.
To determine whether the sterol-linked inhibitor was in fact reaching endosomes, the scientists made fluorescent derivatives of both the sterol-linked and the free inhibitor and visualized treated cells under the microscope. These experiments showed not only that the membrane-tethered inhibitor was getting endocytosed, but also that concentrations as low as 0.1 micromolar could block the appearance of the β-cleaved APP ectodomain in the cells. Disrupting endocytosis with a mutant dynamin greatly reduced internalization of the inhibitor, they found.
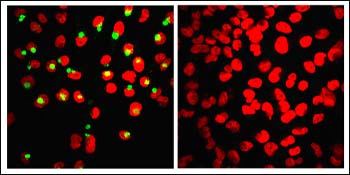
The left image shows production of β-cleaved APP ectodomain (green) as an indication of β cleavage in control cells, whereas treatment with the membrane-anchored inhibitor abolishes production of the β-cleaved ectodomain (right image). Nuclei appear in red. Image credit: Lawrence Rajendran
Given that specialized, cholesterol-rich membrane domains called lipid rafts are enriched in β-secretase and function as sites of APP cleavage (Cordy et al., 2006), Rajendran and colleagues asked whether the potency of their sterol-linked inhibitor was related to how effectively it was targeted to lipid rafts. To this end, they created inhibitors with different anchors carrying various membrane and raft affinities. This experiment showed that the more “raftophilic” the anchor, the more it was able to augment the compound’s inhibitory potential.
To show in vivo effectiveness, the researchers tested their sterol-linked inhibitor first in a fruit fly model system, then in mice. The flies are triple transgenic (expressing human APP, β-secretase, and presenilin), develop neurodegeneration with age, and tend to die prematurely. This phenotype is preventable with secretase inhibitors (Greeve et al., 2004). Treating the larvae with the membrane-anchored inhibitor increased their survival rates about three- to sixfold relative to solvent-fed controls. When injected into the hippocampus of APPsw/PSDE9 mice, a transgenic model with early and robust AD pathology (Radde et al., 2006), the sterol-linked compound inhibited hippocampal Aβ levels at least twofold better than did the free inhibitor, which fared just as poorly as the solvent control.
With these findings, the authors rationalize that a membrane-anchored version of the inhibitor would be superior to a soluble but membrane-permeable inhibitor. Tethering the inhibitor to the membrane not only got it into endosomes to reach its target, they argue, but it also increased the inhibitor’s local membrane concentration, boosting reaction rates between inhibitor and target. Rajendran and colleagues are collaborating with chemists from Technical University Dresden to apply their membrane-tethering strategy to an HIV fusion inhibitor.
“This is definitely a very innovative and clever approach to drug therapy for Alzheimer disease,” said Corinne Augelli-Szafran, a medicinal chemist with 20 years of drug discovery experience. However, “the greater challenge will be to design these types of inhibitor into having drug-like characteristics,” she told ARF via e-mail (see Q&A below). Augelli-Szafran directs the Laboratory of Experimental Alzheimer Drugs at Brigham and Women’s Hospital, Boston, and was not involved in the new BACE work.
Her sentiment was shared by Jordan Tang, scientific co-founder of CoMentis, Inc., a San Francisco-based company whose β-secretase inhibitor appeared promising in recent human trials of the small-molecule drug (see ARF related Keystone story). Though membrane tethering is an “interesting concept for targeting β-secretase inhibitors to endosomes,” Jordan said via e-mail, a key concern is that the conjugation would make inhibitors considerably larger. “Since the blood-brain barrier has a cut-off size of about 500 Da,” he wrote, “people designing drugs are strained to put selectivity, blood-brain barrier penetration and potency all in a small package.” (See full comment below.)
As a first step toward addressing the blood-brain barrier issue, Rajendran told ARF that experiments are underway to determine whether membrane-anchored inhibitors injected into mouse brains intraventrically can produce effects comparable to direct injections into the hippocampus.—Esther Landhuis
Esther Landhuis is a freelance writer in Dublin, California.
Q&A with Corinne E. Augelli-Szafran. Questions by Esther Landhuis.
Q: Does targeting BACE inhibitors to endosomes make sense from a drug development perspective?
A: This is definitely a very innovative and clever approach to Alzheimer disease therapy. The data clearly support the presented hypothesis. However, this approach is in its infancy in determining whether it can actually reach a point of practical drug therapy. The first impression and available data are such that this strategy may mostly be used mechanistically and as a pharmacological tool for this disease.
Q: What are some of this strategy’s advantages and disadvantages?
A: The advantage is that a novel approach to inhibiting BACE has been confirmed, and this in itself generates excitement in the area of Alzheimer research. These new data clearly support that membrane-anchored inhibitors can be transported to the endosomes, where β cleavage occurs, and the results presented add significant understanding to this therapeutic area.
The disadvantage is that the inhibitors presented are of chemical matter that is non-drug-like in character. Extensive research would need to be done to advance these peptide inhibitors to entities that resembled drug development candidates, before even considering the stage of human clinical trials. The timeframe to reach this point of return seems to be far into the future. Brain penetration, bioavailability, clearance, metabolism, toxicity are among the issues that would need to be addressed.
Q: What have been some of the technical challenges in BACE inhibitor design? Does this paper address any of them?
A: They have been to modify the inhibitors to a point of druggability, meaning to have the necessary pharmacokinetic and brain-penetrant profiles such that significant efficacy is observed in vivo both in animals and humans. Most BACE inhibitors to date have been peptide-like in character and, hence, contribute to the inability to optimize the compounds sufficiently to first-in-human trials. The work presented here shows a completely different type of inhibitor that apparently is able to illustrate greater inhibition because of its membrane-anchoring character that allows the inhibitor to be transported to the endosomes where the β cleavage occurs. But the greater challenge will be to design these types of inhibitors into having drug-like characteristics. This issue was not discussed in this paper, nor was how this strategy could be practically applied to designing drugs. However, it was stated that the general approach could be applied to other membrane protein targets.
Overall, this paper clearly demonstrates classical innovation and convincing, elegant results that advance Alzheimer research, perhaps both mechanistically and as pharmacological tools. However, using these inhibitors as a potential drug discovery approach towards the identification of a novel therapy does not seem practical at this time.
References
News Citations
- Chew ’em Up and Spit ’em Out: Aβ Leaves Cells via Exosomes
- Link Between Synaptic Activity, Aβ Processing Revealed
- Keystone Drug News: CoMentis BACE Inhibitor Debuts
Paper Citations
- Small SA, Gandy S. Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron. 2006 Oct 5;52(1):15-31. PubMed.
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006 Jul 25;103(30):11172-7. PubMed.
- Cordy JM, Hooper NM, Turner AJ. The involvement of lipid rafts in Alzheimer's disease. Mol Membr Biol. 2006 Jan-Feb;23(1):111-22. PubMed.
- Greeve I, Kretzschmar D, Tschäpe JA, Beyn A, Brellinger C, Schweizer M, Nitsch RM, Reifegerste R. Age-dependent neurodegeneration and Alzheimer-amyloid plaque formation in transgenic Drosophila. J Neurosci. 2004 Apr 21;24(16):3899-906. PubMed.
- Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jäggi F, Wolburg H, Gengler S, Haass C, Ghetti B, Czech C, Hölscher C, Mathews PM, Jucker M. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006 Sep;7(9):940-6. PubMed.
Other Citations
External Citations
Further Reading
Primary Papers
- Rajendran L, Schneider A, Schlechtingen G, Weidlich S, Ries J, Braxmeier T, Schwille P, Schulz JB, Schroeder C, Simons M, Jennings G, Knölker HJ, Simons K. Efficient inhibition of the Alzheimer's disease beta-secretase by membrane targeting. Science. 2008 Apr 25;320(5875):520-3. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Oklahoma Medical Research Foundation
This work described an interesting concept for targeting β-secretase to endosomes. The results show some promise. However, the improvement in the inhibition of Aβ production by sterol-modified inhibitors over the unmodified inhibitor was accomplished with an early-stage inhibitor. Although the inhibitor structure was not revealed in the paper and supplements, the fact that the inhibitor was synthesized by automated peptide synthesizer suggests that it may be similar to the first inhibitor OM99-2 we described in 2000 (Hong et al., 2000; Lin et al., 2000).
The first-generation peptidic inhibitors have poor ability to penetrate membranes. Thus, the sterol modification would help their activity in cells and in vivo. However, the late-generation inhibitors are no longer peptidic and are relatively small. Their ability to penetrate membranes and inhibit Aβ production is well demonstrated now in transgenic mice (both i.v. and oral) and in human clinical trials (by CoMentis).
It is not clear that the membrane-anchoring modification would help develop better β-secretase inhibitor drugs. Of special concern is that the conjugation makes inhibitors considerable larger. Since the blood-brain barrier has a cutoff size of about 500 Da, people designing drugs are strained to put selectivity, blood-brain-barrier penetration, and potency all in a small package. The sterol conjugation does not seem to help blood-brain barrier penetration, as the transgenic experiment was done with direct injection of the conjugated inhibitor into the brain, thus bypassing the blood-brain barrier.
On the scientific side, the results do demonstrate that a membrane-anchored inhibitor can inhibit β-secretase, which suggests that the protease has considerable freedom of gyration in endosomes. This is good to know.
References:
Hong L, Koelsch G, Lin X, Wu S, Terzyan S, Ghosh AK, Zhang XC, Tang J. Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science. 2000 Oct 6;290(5489):150-3. PubMed.
Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc Natl Acad Sci U S A. 2000 Feb 15;97(4):1456-60. PubMed.
Indiana University School of Medicine
This work clearly demonstrates the importance of targeting to the right subcellular localization in the inhibition of BACE.
To test if a BACE inhibitor can reduce Aβ production in vivo, the authors used the APPswe/PS mutant transgenic mouse model. It is unclear exactly to me which mouse model was used in the current study. Although it was stated in the text that APPswe/PS1delta9 mice were used, an article describing APPswe/PS1L166P transgenic mice was cited.
If APPswe/PS1Δ9 mice were used, it might be still reasonable to test the effect of the BACE inhibitor after four hours of injection, given the very low plaque load at four to five months of age in APPswe/PS1Δ9 mice.
However, if APPswe/PS1L166P mice were used, their huge plaque load at four to five months of age might confound the interpretation, even with PBS extraction and a bead grinder homogenizer.
University of Zurich
I have to thank Alzforum's new reporter, Esther Landhuis, for the coverage on our recent study. Great work!
The comment by Drs. Tang and Augelli-Szafran on the non-drug-likeness of our inhibitor is extremely important. Membrane anchoring of an inhibitor requires the presence of a lipid-anchor and also a linker molecule, and hence, considerably increases the size of the inhibitor. Size definitely matters when it comes to crossing the blood-brain barrier. We also share the same concern these commentators have as to whether our drug can pass the BBB. We are currently performing mice experiments in collaboration with Mikael Simons from the Max Planck Institute in Goettingen to see if the inhibitor, when administered through several different routes, can pass the BBB.
Having said that, I want to emphasize here that we do not claim that we have a “drug” against AD. Our work is more a proof for the principle of subcellular targeting: since much of the active β-secretase is found only in the endosomal compartment, it is important that transition-state inhibitors against β-secretase reach this compartment. We hypothesized that membrane anchoring would render the anchored inhibitor endocytosis-competent and thereby traffic the inhibitor to the endosomes. As a proof-of-principle, we membrane-anchored a non-permeable inhibitor and compared it with the unanchored version of the inhibitor (all structures—parent inhibitor and the modifications—are shown in Supplementary Figure 3). The anchored version was more effective than the free inhibitor in inhibiting β-secretase suggesting that the inhibitors should reach this endosomal compartment in order to effectively inhibit β-secretase. Inhibitors that accomplish this task by permeating through the membrane, thereby gaining access to the endosomal β-secretase, also support the hypothesis.
We are also trying to vary the pharmacophore (currently of peptidic nature) to small-molecule inhibitors of β-secretase along with variations in the linker lengths. This would enable us to formulate a considerably smaller, yet membrane-anchored version of the inhibitor.
Inferring from our Drosophila experiments, one could assume that the sterol-linked inhibitor that was mixed with the feed crossed the gut epithelium via transcytosis to reach other tissues. Since transcytosis is a mechanism by which several drugs cross the blood-brain barrier, it is not impossible that such an inhibitor could also be transported across the blood-brain barrier and be delivered to the brain. Lipoprotein particles could aid in the delivery. These are, of course, assumptions that have to be tested by further work.
One aspect that we couldn’t emphasize much in the paper is that membrane anchoring reduced the dimensionality of the otherwise soluble inhibitor. By membrane anchoring, the inhibitor partitions readily into the membrane plane, thereby increasing the concentration of the inhibitor in the membrane plane. We will now test the reduction in dimensionality principle by membrane anchoring a membrane-permeable inhibitor.
Thanks to Jungsu for pointing out the mistake regarding the nomenclature of the mice—we have written an erratum. Thanks once again to all commentators.
Make a Comment
To make a comment you must login or register.