BACE Block Nips New Plaques in the Bud, Old Ones Keep Growing
Quick Links
Turning down the Aβ spigot may change the future trajectory of amyloid deposition, but it does not turn back time. Using two-photon microscopy to track the “lives” of individual plaques in an AD mouse model, researchers led by Jochen Herms at the German Center for Neurodegenerative Diseases in Munich found that reducing Aβ production with the BACE1 inhibitor NB-360 squashed new plaque formation by 12-fold. However, existing plaques were no pushovers. They continued to grow, albeit at half speed. And they remained toxic, damaging nearby neurons. Published January 11 in Acta Neuropathologica, the study also reports that in the treated mice, BACE1 accumulated within swollen dystrophic axons near existing plaques. There, the enzyme evades the clutches of the inhibitor, allowing local production of Aβ to fuel further plaque growth, claim the authors. In all, the findings suggest that BACE1 inhibition may be most effective at the earliest stages of Aβ deposition, before plaques have formed.
- In mice, BACE1 inhibition decimated formation of new plaques, but only halved the growth of existing ones.
- New plaques appeared mostly near existing plaques, accompanied by swollen axons loaded with BACE1.
- BACE1 inhibition slowed development of dystrophic axons, but did not heal existing damage.
Prior studies were unable to determine if BACE1 inhibition has the power to destroy plaques, shrink them, or merely slow down their growth, because they were cross-sectional in nature. They looked at a single time point or analyzed amyloid burden in different mice at different times (May 2015 news; Nov 2005 news; Jacobsen et al., 2014). To directly address how BACE1 inhibition affects both the number and growth of plaques, first author Finn Peters and colleagues turned to a tool that has become the bread and butter of the Herms lab: longitudinal, live, two-photon imaging. The researchers permanently affixed cranial windows atop the somatosensory cortices of APP/PS1 x VGLUT1-Venus mice. These hybrid animals aggressively develop Aβ plaques and also have fluorescent boutons to allow monitoring of axonal damage, courtesy of a glutamine transporter-fluorescent protein chimera.
The researchers treated the mice with Novartis’s NB-360, a BACE1 inhibitor that penetrates the brain. They started feeding mice the inhibitor at four months of age, during the early phase of Aβ deposition, and continued for 12 weeks. Every week, they imaged the growth and emergence of plaques—an average of 67 plaques per mouse. Strikingly, the treatment almost entirely prevented formation of new plaques, knocking down their appearance by 12-fold. In contrast, the treatment made but a modest dent in existing plaques, slowing their growth by 52 percent. No plaques shrank or disappeared throughout the treatment period.
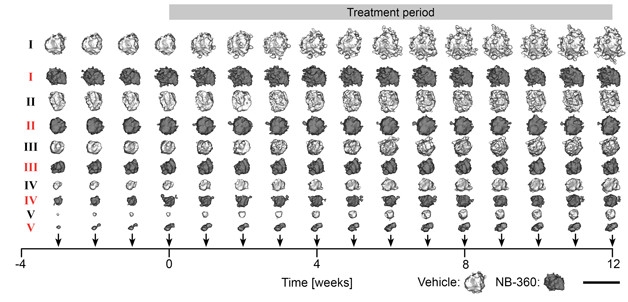
Slowing but Not Shrinking. Comparing sized-matched (I – V) pairs of plaques shows that NB-360 slowed the growth of plaques (dark gray) relative to vehicle (light gray), regardless of their starting size. [Courtesy of Peters et al., Acta Neuropathologica, 2018.]
Given the inhibitor’s differential effect on new plaque versus old, the researchers next examined the spatial relationships between the two. First, they determined that new plaques were far more likely to spring up in the vicinity of existing ones. Even in mice treated with a BACE1 inhibitor, the few new plaques that emerged only did so within 40 μm of an existing plaque. This suggested to Herms and colleagues that the environment surrounding plaques was most welcoming to newbies.
Moreover, the scientists observed accumulating BACE1 crowding the swollen, dystrophic axons surrounding existing plaques (see image below), and the intensity of this accumulation increased in proportion to plaque size. They proposed that not only might these BACE1-laden axons pump out the Aβ that fuels the growth of the nearby existing plaque, but it could also yield enough Aβ to trigger a brand-new one. The high concentration of BACE1 in damaged axons could set a higher stoichiometric hurdle for BACE inhibitors, they believe. This would allow enough localized BACE activity for the continued growth of existing plaques in treated mice, but not enough for new plaques, which can only be kick-started at higher concentrations of Aβ, posit the authors.

Halo of Havoc.
Clusters of BACE1 accumulation (pink) circled the surface of Aβ plaque (blue). Treatment with NB-360 (right) did not affect this plaque-associated BACE1 accumulation. [Courtesy of Peters et al., Acta Neuropathologica, 2018.]
What about downstream of Aβ and plaques? Peters and colleagues next assessed whether reduction in the rate of Aβ deposition would reverse or reduce axonal damage. Using VGLUT1-Venus fluorescence to measure the size and presence of presynaptic boutons, the researchers tracked the appearance of swollen dystrophic axons, which they categorized as those larger than 2 μm in diameter. They found that the dystrophies emerged around plaques as small as 4 μm in diameter, and increased in number as plaques grew, maxing out when plaques reached 10–14 μm in diameter. At the same time, the number of healthy boutons nearby plummeted.
BACE1 inhibition did not disrupt the association between existing plaques and axonal damage—individual plaques of a given size still evoked the same amount of nearby axonal dystrophy as in untreated mice. However, because NB-360 blocked the formation of new plaques and slowed the growth of existing ones, it reduced the appearance and growth of axonal dystrophies, resulting in 10-fold less damage in treated mice at the end of the treatment period.
The findings support the idea that BACE1 inhibition slows progression of Aβ pathology, but cannot shrink existing plaques nor lessen the damage already done to nearby neurons, Herms told Alzforum. “Plaques, once formed, create an environment that favors more Aβ deposition,” Peters added.
Given the strong effect of BACE1 inhibition on preventing new plaques, Peters and Herms said the data supports the idea that this treatment must begin before or during the early stages of Aβ deposition, when new plaques are still emerging. This could explain why Merck’s BACE inhibitor, verubecestat, failed to slow decline in people with mild to moderate AD in Phase 3 (Dec 2017 conference news). Furthermore, given the accumulation of BACE1 in axonal dystrophies, higher doses of inhibitor may be needed to effectively inhibit the enzyme in a brain ravaged by this pathology, than in someone who has very little amyloid in his or her brain. On the flip side, this could mean that much lower doses of the drug could be effective at earlier stages of the disease, they said.
“This work is beautiful and comprehensive in quantitating plaque growth and formation,” commented Randall Bateman of Washington University in St. Louis. “Peters et al. clearly demonstrated better efficacy of the β-secretase inhibitor before individual plaque formation, suggesting that treating during a period of new plaque formation, or even before, will be most effective. This would translate to the 10 to 20 years before symptom onset in DIAN,” he wrote to Alzforum. Indeed, DIAN investigators are planning to treat asymptomatic carriers of autosomal-dominant AD mutations with a BACE inhibitor during this time window. Other prevention trials, including the EARLY trial and API’s Generation Study, which target Aβ-positive and ApoE4 homozygote cognitively normal people, respectively, also aim at the presymptomatic phase of the disease, but the level of Aβ deposition is likely to vary among participants.
Stefan Lichtenthaler, Herms’ neighbor at DZNE, agreed with Bateman. “This is a beautiful and carefully conducted study which emphasizes the view that BACE inhibitors—in order to be really effective—need to be given early in the pathogenesis,” he wrote to Alzforum. “Translated to patients, this means that BACE inhibitors need to be tested at the presymptomatic stage, which is in excellent agreement with other studies, including recent Phase 3 clinical trials.”
Robert Vassar of Northwestern University in Chicago also agreed, but interpreted the data a bit differently. He pointed out that the effect of BACE1 inhibition on slowing the growth of existing plaques is nothing to sneeze at, and clearly prevents progression of pathology. Therefore, treatment could still be beneficial if given after most plaques have formed, but are still growing. “Clearly beyond the plateau is too late, but as you go down that curve, who knows?” He said that ongoing trials in presymptomatic people and those with MCI could answer that question.
Joanna Jankowsky of Baylor College of Medicine in Houston echoed Vassar’s positive take. “It’s not too late until the neurons are dead,” she told Alzforum. She added that in lieu of starting BACE1 inhibition decades prior to symptom onset, it may be feasible to treat people who test positive for Aβ accumulation with a combination of an Aβ antibody and a BACE inhibitor. Then, following the removal of existing plaques, patients could be maintained on a low dose of BACE inhibitor. This approach is promising given previous studies indicating that plaque clearance via Aβ antibodies heals axonal dystrophies, which would reduce this source of highly concentrated BACE1 and Aβ in the brain, Jankowsky and Vassar pointed out (Jan 2005 news).—Jessica Shugart
References
News Citations
- Do BACE Inhibitors Activate Microglia?
- SfN: How to Dispose of Plaques? Closing Spigot Won’t Do; Enzymes Nibble
- Verubecestat Negative Trial Data: What Does it Mean for BACE Inhibition?
- Window to the Brain Shows Dystrophic Neurites Shrinking
Research Models Citations
Therapeutics Citations
Paper Citations
- Jacobsen H, Ozmen L, Caruso A, Narquizian R, Hilpert H, Jacobsen B, Terwel D, Tanghe A, Bohrmann B. Combined treatment with a BACE inhibitor and anti-Aβ antibody gantenerumab enhances amyloid reduction in APPLondon mice. J Neurosci. 2014 Aug 27;34(35):11621-30. PubMed.
Further Reading
Primary Papers
- Peters F, Salihoglu H, Rodrigues E, Herzog E, Blume T, Filser S, Dorostkar M, Shimshek DR, Brose N, Neumann U, Herms J. BACE1 inhibition more effectively suppresses initiation than progression of β-amyloid pathology. Acta Neuropathol. 2018 May;135(5):695-710. Epub 2018 Jan 11 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University School of Medicine
The work by Peters et. al is beautiful and comprehensive in quantitation of plaque growth and formation. They clearly demonstrated better efficacy of the β-secretase inhibitor before individual plaque formation, suggesting that treating during a period of new plaque formation, or even before, will be most effective. This would translate to the 10–20 years before symptom onset in DIAN.
A caveat of the work is that the mouse model overexpresses Aβ production, so the concentrations and kinetics may not replicate typical human plaque growth.
Make a Comment
To make a comment you must login or register.