In AD, CSF Immune Cells Hint at Mounting Mayhem in the Brain
Quick Links
Immune cells within the cerebrospinal fluid can offer a glimpse of the inflammatory conditions within the brain. According to a study published December 7 in Cell, those immune cells markedly change gene expression as people round out their eighth decade—monocytes ramp up expression of lipid transport genes, including ApoE, while they dampen pro-inflammatory cytokine expression. However, in MCI or AD, CSF monocytes do the opposite, turning down lipid transport genes. Instead, they crank up expression of CXCL16, a chemokine that may beckon cytotoxic T cells to the brain. The same may go on deep in the parenchyma. Microglia congregating around plaques expressed the same chemokine, and T cells expressing its receptor, CXCR6, joined them at the scene, report the authors. Together, the findings suggest patterns of immune dysfunction in older healthy people are distinct from those in neurodegenerative disease. How these cells contribute to the pathogenesis in the latter remains an open question.
- In people’s 70s, immune cells of the CSF become less inflammatory.
- In AD, the opposite occurs, as monocytes crank out the chemokine CXCL16.
- Cytotoxic T cells expressing the CXCR6 receptor respond.
- In AD parenchyma, CXCL16-expressing microglia and CXCR6+ T cells surround plaques.
The CSF is bustling with immune cells. Recently, scientists found that as these migrate through the meninges and flush through the brain’s lymphatic system, they may communicate with cells in the parenchyma (Da Mesquita et al., 2018; May 2021 news). As a postdoc in Tony Wyss-Coray’s lab at Stanford, David Gate previously detected a glut of cytotoxic CD8+ T cells within the CSF and brain tissue of people with AD and PD (Jan 2020 news). These killer cells had clonally expanded, meaning they had multiplied after encountering their cognate antigen. Similarly, these scientists later reported an abundance of CD4+ T cells in the CNS of people with dementia with Lewy bodies, where the cells encircled the α-synuclein-laden deposits (Oct 2021 news). Together with reports from other labs, these findings help build the case that T cells are involved in neurodegenerative disease.
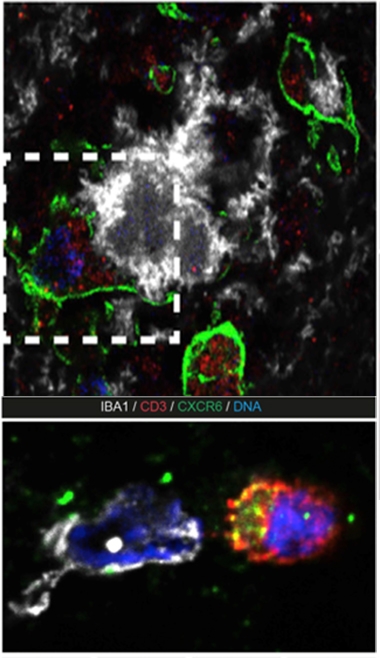
Call of the Chemokine. In the top panel, microglia (green) near plaques (white) express abundant CXCL16 (red). In bottom panel, T cells (red) expressing the CXCR6 receptor (green) are recruited to the scene, where they engage with microglia (white). [Courtesy of Piehl et al., PNAS, 2022.]
Continuing this line of research at his lab at Northwestern, Gate investigated how immune cells in the CSF change with age and with neurodegenerative disease. To look at age-related changes, first author Natalie Piehl and colleagues used single-cell RNA sequencing to survey the transcriptomes of immune cells in CSF collected from 45 cognitively normal people who ranged from 54 to 82 years of age. The samples were collected at AD research centers at Stanford, UCSF, and UCSD. The analysis detected all manner of immune cell types, including T cells, T-regulatory cells, natural killer cells, plasma cells, B cells, and other sorts of monocytes, the cells of the innate immune system. The relative proportion of these cell types did not differ by age of the donor, and the most common cell types in the CSF were CD4+ and CD8+ T cells.
The transcriptomes of T cells and non-classical monocytes—defined by their expression of CD14 and CD16 and their patrol of the vasculature—did change with age of the donor, however. While markers of activation increased in T cells, non-classical monocytes downregulated genes coding pro-inflammatory cytokines and chemokines. Notably, these monocytes expressed ever-higher levels of lipid transport genes with age, including the AD risk genes ApoE and ApoC1, as well as phospholipid transfer protein, which regulates lipid metabolism and influences T cell responses. These differences in gene expression peaked at age 78.
The researchers next surveyed CSF transcriptomes of eight people with a clinical diagnosis of MCI and six with AD. Most had higher concentrations of p-tau181 in their CSF than did controls. T-regulatory cells were most heavily perturbed, with a strong uptick in the FoxP3 transcription factor that drives their anti-inflammatory phenotype. Non-classical monocytes also turned down expression of lipid processing genes with age, in contrast to the uptick in healthy older people. Gate told Alzforum that, at the transcriptional level, these monocytes resembled lipid-accumulating microglia (LAM) identified in a previous study from Wyss-Coray’s lab. Stuffed with lipid droplets, these microglia churned out inflammatory cytokines and reactive oxygen species (Aug 2019 news). While scientists have yet to reach a solid consensus on the functional characteristics that distinguish non-classical from classical and intermediate monocytes, the non-classical reportedly patrol the vasculature, respond to injury, and present antigens to T cells (Thomas et al., 2015; Marsh et al., 2017).
How might these gene expression changes influence the way immune cells communicate with each other? To find out, the researchers used CellChat, a program that infers cell-cell interactions from snRNA-Seq data. Regardless of cognitive status, interactions between non-classical monocytes and CD8+ T cells increased markedly with age across the CSF samples. However, one communication line stood out in MCI/AD: CXCL16-CXCR6 signaling. Specifically, non-classical monocytes expressed the chemokine CXCL16, while CD8+ T cells revved up expression of its receptor, CXCR6. Notably, the researchers determined that the strongest CXCR6 expression came from T cells that had clonally expanded, indicating that they had previously seen their cognate antigen and multiplied in response. What those antigens are, was not determined. These were predominantly T effector memory cells. TEM are experienced killers, rapidly dispensing with any cell presenting their cognate antigen, and spewing out a slew of pro-inflammatory cytokines in the process. They were also identified in Gate’s previous study in people with AD and PD.

Immune Shifts with Age, AD. CSF sampling of healthy and MCI/AD volunteers revealed distinct age and disease-related changes in the transcriptomes of immune cells. CXCL16-CXCR6 signaling increased with age only among the cognitively impaired. [Courtesy of Piehl et al., Cell, 2022.]
In a larger set of CSF samples, the researchers found elevated CXCL16 among people who were cognitively impaired. Notably, the CSF concentration of this chemokine correlated with neurodegenerative biomarkers neurofilament light (NfL) and glial fibrillary acidic protein (GFAP). Among those with MCI who progressed to AD, CXCL16 tracked with p-tau181, but curiously not in those diagnosed with full AD.
Might the CXCL16-CXCR6 pathway also be active within brain tissue? Indeed, Piehl found that in people with AD, plaque-associated microglia expressed abundant CXCL16, and CXCR6-expressing T cells lurked nearby. Strikingly, CXCR6+ T cells extended processes that touched plaques, suggesting they were actively engaged with the aggregates in some way.
In all, the findings suggest that microglia responding to plaques may inadvertently recruit killer CXCR6+ T cells to the scene, Gate said. What happens after that remains unclear. However, given their known cytotoxicity, Gate hypothesized that these T cells spell trouble for neurons. This idea jibes with a recent report that the same chemokine pathway maintains cytotoxic T cells in the brain following West Nile virus infection (Rosen et al., 2022). The lingering T cells destroyed synapses, and the scientists suggested they cause the cognitive impairment many people experience post infection.
“Because CXLCL16 is produced by myeloid cells in the brain, including microglia, the data suggest that microglial secretion of CXCL16 is a homing signal for clonally expanded CD8 T cells to come into the brain,” wrote David Holtzman of Washington University in St. Louis (comment below). “While these data don’t prove that these interesting changes are causal for AD pathogenesis, neurodegeneration, and disease progression, experiments should be done to determine the contribution of these molecules and clonally expanded T cells to neurodegeneration in AD.”—Jessica Shugart
References
News Citations
- As Mice Age, T Cells Traipse Around Their Meninges. Mayhem Ensues
- Attack of the Clones? Memory CD8+ T Cells Stalk the AD, PD Brain
- Intruder Alert: Inflammatory T Cells Lurk Near Lewy Bodies, Neurons
- Newly Identified Microglia Contain Lipid Droplets, Harm Brain
Paper Citations
- Da Mesquita S, Fu Z, Kipnis J. The Meningeal Lymphatic System: A New Player in Neurophysiology. Neuron. 2018 Oct 24;100(2):375-388. PubMed.
- Thomas G, Tacke R, Hedrick CC, Hanna RN. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol. 2015 Jun;35(6):1306-16. Epub 2015 Apr 2 PubMed.
- Marsh SA, Arthur HM, Spyridopoulos I. The secret life of nonclassical monocytes. Cytometry A. 2017 Nov;91(11):1055-1058. Epub 2017 Oct 27 PubMed.
- Rosen SF, Soung AL, Yang W, Ai S, Kanmogne M, Davé VA, Artyomov M, Magee JA, Klein RS. Single-cell RNA transcriptome analysis of CNS immune cells reveals CXCL16/CXCR6 as maintenance factors for tissue-resident T cells that drive synapse elimination. Genome Med. 2022 Sep 24;14(1):108. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Piehl N, van Olst L, Ramakrishnan A, Teregulova V, Simonton B, Zhang Z, Tapp E, Channappa D, Oh H, Losada PM, Rutledge J, Trelle AN, Mormino EC, Elahi F, Galasko DR, Henderson VW, Wagner AD, Wyss-Coray T, Gate D. Cerebrospinal fluid immune dysregulation during healthy brain aging and cognitive impairment. Cell. 2022 Dec 22;185(26):5028-5039.e13. Epub 2022 Dec 13 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University
In this interesting resource paper by Piehl et al. from the lab of David Gate, the authors assess the CSF immune cell profiles of 45 cognitively normal subjects and 14 cognitively impaired subjects who likely had very mild to mild Alzheimer’s disease. Using single-cell RNA-Seq, they found that populations of monocytes upregulated lipid transport genes with age. In those participants who were cognitively impaired, there was a relative downregulation of lipid transport genes in monocytes with features of altered cytokine signaling that would affect T cells. Interestingly, there was an increase in clonal CD8 T cells in CSF of those with cognitive impairment, as well as an increase in CXCR6 in these cells along with an elevation of the CXCR6 ligand CXCL16 in the CSF.…More
Because CXLCL16 is produced by myeloid cells in the brain, including microglia, the data suggest that microglial secretion of CXCL16 is a homing signal for clonally expanded CD8 T cells to come into the brain. While these data don’t prove that these interesting changes are causal for AD pathogenesis, neurodegeneration, and disease progression, they suggest that experiments should be done to determine the role of these molecules and clonally expanded T cells and their contribution to neurodegeneration in AD.
Weizmann Institute of Science
The role of the immune system in shaping the brain’s fate in health, aging, and disease has received significant attention over the last two decades (Croese et al., 2021). Yet, understanding the detailed pathology of these diseases is still in its infancy. The roles of the choroid plexus (CP) and the cerebrospinal fluid (CSF) have received greater focus with the understanding that the CP is an important immune-brain interface (Shechter et al., 2013; Baruch et al., 2014), and with the discovery that the CSF, via the microchannels that connect the brain to the skull bone marrow (BM), flushes the BM and thereby affects the BM cells (Mazzitelli et al., 2022; Herisson et al., 2018). Yet, the fate of the CSF is also affected by the brain milieu, by the CP, and by the circulating immune cells. This entire immunological network creates an ecosystem (Schwartz et al., 2022), and therefore any component that goes awry in this network could contribute to a vicious cycle of neurodegeneration (Croese et al., 2021; Da Mesquita et al., 2021; Goldman et al., 2022; Minhas et al., 2021). Accordingly, the immunological profile of the CSF could potentially be used as a proxy for the fate of this network.…More
In the present study, the authors describe results that highlight the potential of utilizing CSF immune transcriptome changes to identify disease-associated neuroinflammation in cognitively impaired individuals; such CSF immunophenotyping may be useful to gain further insight into T cell-antigen complexes involved in the pathophysiology of cognitive impairment. The data provided in this article could be used as a resource in studies aimed at disease characterization, or those identifying markers of therapeutic effects.
References:
Croese T, Castellani G, Schwartz M. Immune cell compartmentalization for brain surveillance and protection. Nat Immunol. 2021 Sep;22(9):1083-1092. Epub 2021 Aug 24 PubMed.
Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013 Mar 21;38(3):555-69. Epub 2013 Mar 7 PubMed.
Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, Amit I, Schwartz M. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014 Aug 21; PubMed.
Mazzitelli JA, Smyth LC, Cross KA, Dykstra T, Sun J, Du S, Mamuladze T, Smirnov I, Rustenhoven J, Kipnis J. Cerebrospinal fluid regulates skull bone marrow niches via direct access through dural channels. Nat Neurosci. 2022 May;25(5):555-560. Epub 2022 Mar 17 PubMed.
Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, Vandoorne K, Wojtkiewicz GR, Masson GS, Vinegoni C, Kim J, Kim DE, Weissleder R, Swirski FK, Moskowitz MA, Nahrendorf M. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci. 2018 Aug 27; PubMed.
Schwartz M, Abellanas MA, Tsitsou-Kampeli A, Suzzi S. The brain-immune ecosystem: Implications for immunotherapy in defeating neurodegenerative diseases. Neuron. 2022 Nov 2;110(21):3421-3424. Epub 2022 Sep 22 PubMed.
Da Mesquita S, Herz J, Wall M, Dykstra T, de Lima KA, Norris GT, Dabhi N, Kennedy T, Baker W, Kipnis J. Aging-associated deficit in CCR7 is linked to worsened glymphatic function, cognition, neuroinflammation, and β-amyloid pathology. Sci Adv. 2021 May;7(21) Print 2021 May PubMed.
Goldman DH, Dykstra T, Smirnov I, Blackburn SM, Da Mesquita S, Kipnis J, Herz J. Age-associated suppression of exploratory activity during sickness is linked to meningeal lymphatic dysfunction and microglia activation. Nature Aging 2: Aug 2022 Nature Aging
Minhas PS, Latif-Hernandez A, McReynolds MR, Durairaj AS, Wang Q, Rubin A, Joshi AU, He JQ, Gauba E, Liu L, Wang C, Linde M, Sugiura Y, Moon PK, Majeti R, Suematsu M, Mochly-Rosen D, Weissman IL, Longo FM, Rabinowitz JD, Andreasson KI. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature. 2021 Feb;590(7844):122-128. Epub 2021 Jan 20 PubMed.
Vrije Universiteit Brussel
Cerebrospinal fluid (CSF) flows through the cerebral ventricles and interconnects the brain parenchyma with its various border tissues. This unique colorless liquid, which also harbors various immune cell types, may hold many clues to overall brain health. The advent of single-cell, multi-omic technologies now offers the prospect of correlating changes in the aging or diseased brain to changes in the CSF immune landscape.
This interesting study, led by David Gate and colleagues, profiled the CSF immune compartment of 59 human subjects via single-cell RNA sequencing, thereby providing a great resource for the field. The results reveal age- or disease-dependent alterations in CSF immune cells, mostly in T cells and a myeloid subset that was identified as nonclassical monocytes. Interestingly, the gene-expression changes observed in these myeloid cells primarily relate to lipid metabolism, which is emerging as a key dysregulated process in many human neurodegenerative disorders. Furthermore, CSF myeloid cells expressed CXCL16, potentially attracting CXCR6-expressing CD8+ T cells to the diseased brain.…More
As discussed in our recent review, the CSF myeloid compartment is quite unique (Munro et al., 2022). Besides monocytes and dendritic cells, the CSF also harbors macrophages. CSF macrophages can be attached to tissue surfaces, an important example being Kolmer’s epiplexus cells, which are microglia-like cells that reside on the apical surface of the choroid plexus epithelium (van Hove et al., 2019). Intriguingly, the CSF also contains freely floating macrophages. In fact, the subset that was identified as nonclassical monocytes by Piehl, Gate, and colleagues likely consists of macrophages. The nice data portal that was developed by the authors allows for an easy inspection of gene expression in the various clusters. This shows that the cluster identified as nonclassical monocytes exhibits negative or low expression of signature genes observed either in all human monocytes, such as FCN1, or genes that are enriched in nonclassical monocytes, such as CDKN1C (Pombo Antunes et al., 2021). Additionally, these cells express high levels of CD14, while nonclassical monocytes are known to exhibit low levels of CD14 (lower as compared to classical monocytes). Furthermore, the cells express high levels of signature macrophage markers, such as C1QA, APOE, and APOC1. Finally, the cells also express microglia/BAM-related genes such as SLC2A5, SPP1, CH25H and TMEM119, which are normally not expressed in nonclassical monocytes, but are found in CSF macrophages (Munro et al., 2022). Therefore, this cluster consists of macrophages and not monocytes. This interpretation is also in line with a recent report that integrated multiple human CSF single-cell datasets (Ostkamp et al., 2022).
This proposed change in nomenclature does not affect any of the key conclusions of the manuscript by the Gate lab, it merely highlights the interesting finding that brain aging and disease significantly affect CSF macrophages, a cell population that has remained poorly understood. For example, how these freely floating CSF macrophages relate to parenchymal microglia and border-associated macrophages remains an open question. Thanks to the current study we now know that CSF macrophages are implicated in healthy and pathological brain aging, which warrants further investigation into the biology and functional significance of this enigmatic brain macrophage subset.
References:
Munro DA, Movahedi K, Priller J. Macrophage compartmentalization in the brain and cerebrospinal fluid system. Sci Immunol. 2022 Mar 4;7(69):eabk0391. PubMed.
Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, De Prijck S, Vandamme N, De Schepper S, Van Isterdael G, Scott CL, Aerts J, Berx G, Boeckxstaens GE, Vandenbroucke RE, Vereecke L, Moechars D, Guilliams M, Van Ginderachter JA, Saeys Y, Movahedi K. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019 Jun;22(6):1021-1035. Epub 2019 May 6 PubMed.
Pombo Antunes AR, Scheyltjens I, Lodi F, Messiaen J, Antoranz A, Duerinck J, Kancheva D, Martens L, De Vlaminck K, Van Hove H, Kjølner Hansen SS, Bosisio FM, Van der Borght K, De Vleeschouwer S, Sciot R, Bouwens L, Verfaillie M, Vandamme N, Vandenbroucke RE, De Wever O, Saeys Y, Guilliams M, Gysemans C, Neyns B, De Smet F, Lambrechts D, Van Ginderachter JA, Movahedi K. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci. 2021 Apr;24(4):595-610. Epub 2021 Mar 29 PubMed.
Ostkamp P, Deffner M, Schulte-Mecklenbeck A, Wünsch C, Lu IN, Wu GF, Goelz S, De Jager PL, Kuhlmann T, Gross CC, Klotz L, Meyer Zu Hörste G, Wiendl H, Schneider-Hohendorf T, Schwab N. A single-cell analysis framework allows for characterization of CSF leukocytes and their tissue of origin in multiple sclerosis. Sci Transl Med. 2022 Nov 30;14(673):eadc9778. PubMed.
The University of Tokyo
CSF, a biological fluid in direct contact and exchange with the central nervous system, has been widely used to study biochemical biomarkers that reflect pathological conditions in the brain. Recent studies on immune cell profiles in CSF have revealed disease-specific changes in immune cell responses, which has led to a better understanding of CSF as an immune system linked to inflammatory responses in the brain. In this study, the authors compared gene expression profiles of CSF immune cells in cognitively normal and cognitively impaired humans of various ages by single-cell RNA-Seq analysis. The authors found gene expression variations in lipid transport and CXCL16-CXCR6 signaling pathways.…More
CXCL16, which has both chemokine activity and scavenger receptor function, has been reported to be involved in tissue distribution and functional changes of innate lymphocytes in peripheral organs. CXCL16-CXCR6 signaling is also known to influence immune-system cell responses in various inflammatory diseases such as atherosclerosis, psoriasis, and tumors (Satoh-Takayama et al., 2014; Favaro et al., 2022; Mabrouk et al., 2022). The possibility that it is involved in signaling between microglia, which are brain-resident immune cells, and infiltrating T cells in the brain is interesting from this point of view. An important question to address is what molecular mechanisms affect the changes in the CXCL16-CXCR6 system during aging and cognitive decline.
References:
Satoh-Takayama N, Serafini N, Verrier T, Rekiki A, Renauld JC, Frankel G, Di Santo JP. The chemokine receptor CXCR6 controls the functional topography of interleukin-22 producing intestinal innate lymphoid cells. Immunity. 2014 Nov 20;41(5):776-88. Epub 2014 Nov 6 PubMed.
Favaro RR, Phillips K, Delaunay-Danguy R, Ujčič K, Markert UR. Emerging Concepts in Innate Lymphoid Cells, Memory, and Reproduction. Front Immunol. 2022;13:824263. Epub 2022 Jun 14 PubMed.
Mabrouk N, Tran T, Sam I, Pourmir I, Gruel N, Granier C, Pineau J, Gey A, Kobold S, Fabre E, Tartour E. CXCR6 expressing T cells: Functions and role in the control of tumors. Front Immunol. 2022;13:1022136. Epub 2022 Oct 12 PubMed.
Make a Comment
To make a comment you must login or register.