In AD, Chaperones Congregate To Form a Therapeutic Target
Quick Links
A new study reveals a sticky web of protein-protein interactions, centered on heat shock protein 90 (HSP90) and other chaperones, in the brains of people with AD. This network, not found in healthy tissue, appears to entangle a host of proteins that are normally involved in synaptic connectivity, learning, and memory. An HSP90 inhibitor called PU-AD, currently in early clinical trials for AD, breaks up the party, normalizing HSP90’s connections and improving synaptic function and cognition in mouse models of tauopathy or AD. The paper, from Gabriela Chiosis, Memorial Sloan Kettering Cancer Center, and Wenjie Luo, Weill Cornell Medical College, both in New York, appeared in Nature Communications on January 16.
The idea that inhibiting HSP90 could mitigate tau toxicity and improve cognition has been around for years (May 2007 news; Wang et al., 2016). However, prior efforts to target HSP90 to treat a different disease—cancer—revealed the blocking the actions of this ubiquitous protein can be quite toxic (Butler et al., 2015). The Chiosis lab discovered that PU-AD and related HSP90 inhibitors bind with high affinity to HSP90-containing protein complexes found in cancer cells, but not normal cells. This raised the possibility of selectively blocking the chaperone only in sick cells (Moulick et al., 2011).
Using their inhibitors as affinity probes, Chiosis and colleagues had previously demonstrated that HSP90 from normal cells co-purified with only a few proteins, but in cancer cells its partners were more numerous and more tightly tethered to each other (Rodina et al., 2016). Chiosis dubbed the cancer-associated complexes the “epichaperome,” to distinguish it from the chaperome—the collection of chaperones, co-chaperones, and associated proteins found in healthy cells.
Working with Lorenz Studer, also at Memorial Sloan Kettering, she later described an analogous epichaperome arising in response to stress in patient-derived neurons from people with Parkinson’s disease (Kishinevsky et al., 2018).
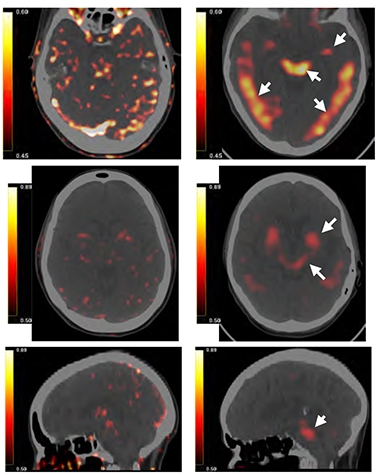
Trouble Spots? PET scans show focal binding of PU-AD to the epichaperome in the bilateral paraphippocampus, temporal lobes, and brainstem (white arrows) of a 70-year old woman with AD (right column). On left, background binding in a 58-year old woman without neurologic disease. [From Inda et al., 2020 Nature Communications.]
In the new study, first authors Maria Carmen Inda, Suhasini Joshi, Tai Wang, and Alexander Bolaender used PU-AD as a chemical probe to characterize the epichaperome in a mouse model of tauopathy. In 3-month-old PS19 mice, the scientists detected binding of radioactive PU-AD mainly in the hippocampus and amygdala, regions associated with learning and memory. In older PS19 mice, PU-AD binding appeared throughout the brain; wild-type mice at any age showed little signal.
Those results were echoed by preliminary live imaging in two people. The scientists observed lower retention of a PU-AD PET ligand in the brain of a cognitively normal 58-year old woman than in a 70-year old woman with AD.
To dissect the epichaperome, the scientists captured HSP90 and associated proteins from control or AD brain extracts using the inhibitor as an affinity probe, and analyzed the complexes this harnessed by mass spectrometry. In normal brain, the inhibitor pulled out proteins predominantly involved in peptide folding and transport. In AD brain, the complexes contained more proteins involved in synaptic formation and function, LTP, memory and learning. Other models—PS19 mice, cells derived from people with AD, and mouse N2a cells overexpressing tau—showed a similar skewing of HSP90-associated proteins.
In cell extracts, PU-AD dissociates the epichaperome, normalizing the proteins partnering with HSP90, the authors showed. Could that help the PS19 tau mice? When the scientists dosed 3-month-old mice for 12 weeks, or 8- to 9-month-old mice for six weeks, the treated mice outperformed untreated mice in a maze test of learning and memory. Treatment normalized deficits in synaptic long-term potentiation as measured in hippocampal slices, and treated mice lived longer. The drug caused no change in HSP90 expression, but it did lead to a decrease in phospho-tau and tau oligomers in multiple brain regions.
PU-AD had similar effects in 3XTg AD mice. In them, it led to a reduction of pathological tau species in the brain, and better memory and learning.
PU-AD is in Phase 1 for clinical development in Alzheimer’s disease. Its maker, Samus Therapeutics, is testing related inhibitors in cancer, and is also developing the PU-AD PET ligand for diagnostic use in cancer and neurologic disease (Pillarsetty et al., 2019).—Pat McCaffrey
References
News Citations
Therapeutics Citations
Research Models Citations
Paper Citations
- Wang B, Liu Y, Huang L, Chen J, Li JJ, Wang R, Kim E, Chen Y, Justicia C, Sakata K, Chen H, Planas A, Ostrom RS, Li W, Yang G, McDonald MP, Chen R, Heck DH, Liao FF. A CNS-permeable Hsp90 inhibitor rescues synaptic dysfunction and memory loss in APP-overexpressing Alzheimer's mouse model via an HSF1-mediated mechanism. Mol Psychiatry. 2016 Jul 26; PubMed.
- Butler LM, Ferraldeschi R, Armstrong HK, Centenera MM, Workman P. Maximizing the Therapeutic Potential of HSP90 Inhibitors. Mol Cancer Res. 2015 Nov;13(11):1445-51. Epub 2015 Jul 28 PubMed.
- Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L, Gomes DaGama EM, Caldas-Lopes E, Beebe K, Perna F, Hatzi K, Vu LP, Zhao X, Zatorska D, Taldone T, Smith-Jones P, Alpaugh M, Gross SS, Pillarsetty N, Ku T, Lewis JS, Larson SM, Levine R, Erdjument-Bromage H, Guzman ML, Nimer SD, Melnick A, Neckers L, Chiosis G. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011 Sep 25;7(11):818-26. PubMed.
- Rodina A, Wang T, Yan P, Gomes ED, Dunphy MP, Pillarsetty N, Koren J, Gerecitano JF, Taldone T, Zong H, Caldas-Lopes E, Alpaugh M, Corben A, Riolo M, Beattie B, Pressl C, Peter RI, Xu C, Trondl R, Patel HJ, Shimizu F, Bolaender A, Yang C, Panchal P, Farooq MF, Kishinevsky S, Modi S, Lin O, Chu F, Patil S, Erdjument-Bromage H, Zanzonico P, Hudis C, Studer L, Roboz GJ, Cesarman E, Cerchietti L, Levine R, Melnick A, Larson SM, Lewis JS, Guzman ML, Chiosis G. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature. 2016 Oct 20;538(7625):397-401. Epub 2016 Oct 5 PubMed.
- Kishinevsky S, Wang T, Rodina A, Chung SY, Xu C, Philip J, Taldone T, Joshi S, Alpaugh ML, Bolaender A, Gutbier S, Sandhu D, Fattahi F, Zimmer B, Shah SK, Chang E, Inda C, Koren J 3rd, Saurat NG, Leist M, Gross SS, Seshan VE, Klein C, Tomishima MJ, Erdjument-Bromage H, Neubert TA, Henrickson RC, Chiosis G, Studer L. HSP90-incorporating chaperome networks as biosensor for disease-related pathways in patient-specific midbrain dopamine neurons. Nat Commun. 2018 Oct 19;9(1):4345. PubMed.
- Pillarsetty N, Jhaveri K, Taldone T, Caldas-Lopes E, Punzalan B, Joshi S, Bolaender A, Uddin MM, Rodina A, Yan P, Ku A, Ku T, Shah SK, Lyashchenko S, Burnazi E, Wang T, Lecomte N, Janjigian Y, Younes A, Batlevi CW, Guzman ML, Roboz GJ, Koziorowski J, Zanzonico P, Alpaugh ML, Corben A, Modi S, Norton L, Larson SM, Lewis JS, Chiosis G, Gerecitano JF, Dunphy MP. Paradigms for Precision Medicine in Epichaperome Cancer Therapy. Cancer Cell. 2019 Nov 11;36(5):559-573.e7. Epub 2019 Oct 24 PubMed.
Further Reading
Papers
- Bolaender A, Zatorska D, He H, Joshi S, Sharma S, Digwal CS, Patel HJ, Sun W, Imber BS, Ochiana SO, Patel MR, Shrestha L, Shah SK, Wang S, Karimov R, Tao H, Patel PD, Martin AR, Yan P, Panchal P, Almodovar J, Corben A, Rimner A, Ginsberg SD, Lyashchenko S, Burnazi E, Ku A, Kalidindi T, Lee SG, Grkovski M, Beattie BJ, Zanzonico P, Lewis JS, Larson S, Rodina A, Pillarsetty N, Tabar V, Dunphy MP, Taldone T, Shimizu F, Chiosis G. Chemical tools for epichaperome-mediated interactome dysfunctions of the central nervous system. Nat Commun. 2021 Aug 3;12(1):4669. PubMed.
Primary Papers
- Inda MC, Joshi S, Wang T, Bolaender A, Gandu S, Koren Iii J, Che AY, Taldone T, Yan P, Sun W, Uddin M, Panchal P, Riolo M, Shah S, Barlas A, Xu K, Chan LY, Gruzinova A, Kishinevsky S, Studer L, Fossati V, Noggle SA, White JR, de Stanchina E, Sequeira S, Anthoney KH, Steele JW, Manova-Todorova K, Patil S, Dunphy MP, Pillarsetty N, Pereira AC, Erdjument-Bromage H, Neubert TA, Rodina A, Ginsberg SD, De Marco Garcia N, Luo W, Chiosis G. The epichaperome is a mediator of toxic hippocampal stress and leads to protein connectivity-based dysfunction. Nat Commun. 2020 Jan 16;11(1):319. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.