Spying on α-Synuclein Inclusions: PET Tracers Inch Closer to Success
Quick Links
PET tracers that illuminate α-synuclein in the brain have been hard to come by, but scientists may be getting closer. According to a study published August 3 in Cell, an 18F- tracer developed at Emory University, Atlanta, binds to α-synuclein aggregates in human Parkinson’s disease brain samples, in mouse models of synucleinopathy, and in nonhuman primates. Crucially, it does so while shunning Aβ plaques or tau tangles, which commonly co-occur with Lewy bodies. Led by Keqiang Ye, now at the Chinese Academy of Sciences in Shenzhen, the study used cryo-electron microscopy to unveil the structural details of the tracer’s specific liaison with α-synuclein. 18F-F0502B joins a growing field of candidate tracers, including three presented at the Alzheimer’s Association International Conference, held July 16-20 in Amsterdam. One, made by AC Immune, binds to α-synuclein aggregates when they are highly concentrated in the brain, including in people with multiple system atrophy and in a genetic form of PD. The company’s other up-and-coming tracer candidate holds promise in latching onto the smaller inclusions that predominant in the PD brain. Another, made by Merck, bound synuclein in mouse models of PD.
- New PET tracer binds α-synuclein aggregates in mice and nonhuman primates.
- Cryo-EM shows it nestles in groove along the fibrils.
- AC Immune and Merck report new candidates at AAIC.
“We are on the cusp of having a selective α-synuclein PET tracer that performs well in Parkinson’s disease,” said Jamie Eberling of the Michael J. Fox Foundation, which contributes funding for several ongoing efforts to develop the tracers, including AC Immune and Merck’s.
A PET ligand for α-synuclein would help immensely with diagnosis and prognosis of synucleinopathies, and with recruitment and monitoring in clinical trials. Yet, suitable tracers have eluded the field for years, due to their subpar affinity for α-synuclein within the brain, as well as off-target binding to other types of aggregates, including Aβ plaques and neurofibrillary tangles. Making matters worse, α-synuclein deposits in the brain can be dwarfed by other protein inclusions. In a landmark for the field last year, AC Immune’s 18F-ACI-12589 detected α-synuclein aggregates in people with multiple system atrophy, but failed to label inclusions in people with other synucleinopathies, including PD (Mar 2022 conference news).
In their hunt, co-first authors Jie Xiang, Youqi Tao, and Yiyuan Xia and colleagues took hints from compounds known to bind and block α-synuclein oligomerization, including molecules with catechol groups, such as dopamine and its derivatives. After screening an initial batch of 23 commercially available compounds for binding to preformed, recombinant α-synuclein fibrils, the researchers ran more extensive screens with derivatives of the top hits. F0502B came out on top. This molecule bound to α-synuclein deposits within brain sections of mice expressing A53T mutant human α-synuclein, but not to Aβ plaques or tau tangles in AD mouse models. F0502B also latched onto α-synuclein in brain sections from people with multiple system atrophy (MSA), dementia with Lewy bodies (DLB), and PD, but far less so to Aβ plaques or tau tangles in AD brain samples. Notably, the molecule, as well as its fluorinated form, 18F-F0502B, bound to α-synuclein from PD or DLB brain sections more tightly than it did to recombinant α-synuclein fibrils.

Path to PET. Chemical candidates, including dopamine derivatives, were screened for specific binding to α-synuclein fibrils (1). Top candidates were further tested in mouse models and in human brain tissue (2). Finally, structure of the α-synuclein/tracer complex was determined, and 18F-F0502B was tested in nonhuman primates (3). [Courtesy of Xiang et al., Cell, 2023.]
To understand how F0502B interacted with α-synuclein fibrils, the researchers turned to cryo-electron microscopy. High-resolution electron density maps revealed the tracer nestled into a deep groove along the surface of recombinant α-synuclein fibrils, which comprised stacked pairs of α-synuclein protofilaments. F0502B filled this binding cavity, stacking along the fibril axis in parallel (image below). While recombinant α-synuclein fibrils are distinct from the “Lewy fold” structures identified in brain samples from people with PD, Parkinson’s disease dementia, and DLB, which are collectively distinct from the fold α-synuclein takes in people with MSA, amino acids forming the F0502B binding pocket are also involved in the Lewy fold (Jul 2022 news). In cryo-EM, this fold is typically occupied by unidentified, nonprotein molecules (Mar 2020 conference news). Further, Ye told Alzforum that only a quarter of the fibrils identified in PD brain samples twisted into the Lewy fold, suggesting substantial structural heterogeneity in fibrils even within a single person. He said his group will discern the structure of tracers in complex with brain derived fibrils.

Finding Fibrils. Cryo-EM image (left) and structural diagram (right) of recombinant α-synuclein fibrils reveal F0502B (orange) binding within a groove formed by stacked pairs of α-synuclein protofibrils. [Courtesy of Xiang et al., Cell, 2023.]
Robert Mach of the University of Pennsylvania in Philadelphia commended the researchers for solving the structure of their tracer in complex with α-synuclein fibrils. He noted that although recombinant fibrils are structurally distinct from those found in the brain, several small-molecule binding sites may be common across fibril isoforms (Hsieh et al., 2018). F0502B binds one of these sites, he said. Still, he thinks one potential concern for F0502B is its dependence on a specific tyrosine residue—Y39—for binding. This residue is a well-known hot spot for phosphorylation and nitration, either of which could influence the structure of the binding pocket.
The scientists put their tracer to the test in rhesus macaques. First, they injected into the striatum preformed, recombinant α-synuclein fibrils, or an adeno-associated virus encoding human A53T-α-synuclein. Eighteen months later, dopamine transporter (DAT) scans of the monkeys revealed substantial nigrostriatal degeneration, akin to that in PD. After infusing the tracer, the researchers found scant retention within the brains of controls, but a significant signal from monkeys burdened with synucleinopathy.
With a standard uptake value ratio of around 0.7, 18F- F0502B may not pass muster as a clinical PET tracer. Rather, Mach views it as a great starting place to make analogs. “We know that very small changes in structure can have a large influence on how these molecules perform in PET imaging studies,” he said. “It is highly possible that a close structural analog could work much better.”
Eberling agreed, noting that the tracer could benefit from further optimization, including improved brain penetrance.
Still, Ye said that although next-generation tracers are under investigation, 18F-F0502B is being tested in people with PD, DLB, and MSA. Braegen Pharmaceuticals, Ltd., a company in Shanghai co-founded by Ye, sponsors development.

Signaling Synucleinopathy. PET scans display 18F-F0502B retention in macaques previously injected with α-synuclein PFFs (bottom left panels) or with AAV-A53T-α-synuclein (right) but not substantially in controls (top left). [Courtesy of Xiang et al., Cell, 2023.]
18F-F0502B joins a handful of other up-and-coming α-synuclein ligands. At AAIC, Francesca Capotosti of AC Immune updated the audience on 18F-ACI-12589, which the company had previously reported to work in people with MSA, but not in other synucleinopathies. Since then, the Capotosti and colleagues, in collaboration with Oskar Hansson at Lund University, Sweden, have scanned more participants, including five with AD, three with PSP, and three with ataxia, as well as 23 with a synucleinopathy. Of the latter group, eight had PD, 13 MSA, and two DLB. For the most part, their initial conclusions held, in that substantial uptake in the brain was seen only in people with MSA. Was the tracer’s penchant for α-synuclein in MSA due to the protein’s conformation, or concentration?
To chip away at this question, the researchers infused the tracer into people with genetic PD or DLB caused by a duplication in the α-synuclein gene. These tend to have a higher burden of α-synuclein pathology relative to people with other forms of PD. Lo and behold, Capotosti did observe ACI-12589 uptake in disease-relevant brain regions, supporting the hypothesis that a high concentration of α-synuclein deposits is needed for them to be detected in the scanner, and that differences in fibril conformation do not explain the tracer’s differential uptake in MSA versus other synucleinopathies. In other words, it’s all about the amount of synuclein, Capotosti said. In support of this idea, 18F-ACI-12589 tracer uptake increased in monkeys after they were inoculated with AAV-A53T-α-synuclein.
Mach has a different interpretation. To his mind, conformational differences in α-synuclein fibrils are still likely to play a strong part in the differential performance of the tracer. Based on screening hundreds of α-synuclein-binding compounds, he said he would be surprised if one worked across all synucleinopathies. “We have radioligands specific for PD, and some for MSA, but not any that bind both,” he said. His group is developing separate tracers for each.
Determined to find a PET agent that can work for PD, AC Immune scientists have developed a new crop of tracers with a higher affinity for α-synuclein aggregates. The top contender, ACI-15916, binds α-synuclein with a fourfold higher affinity than its predecessor. In brain sections from people with PD, the new tracer appeared to detect very small inclusions of α-synuclein, as well as Lewy body neurites, whereas ACI-12589 only latched onto larger inclusions. So far, PET scans in monkeys suggest that the new tracer looks hopeful for clinical studies, Capotosti said.
Idriss Bennacef presented the results of Merck’s quest. He cited low target concentration as the biggest hurdle to success. Merck screened its candidates using brain homogenates from people with PD. Provided by Banner Health, the samples were “clean,” in that they were devoid of Aβ plaques or tau tangles, Bennacef emphasized. Its lead compound—11C-MK-7337—bound tightly and specifically to α-synuclein aggregates in brain sections from people with PD, as well as in A30P-α-synuclein transgenic mice. In PET scans, the tracer lit up regions of the mouse brain that also bound α-synuclein antibodies (image below).
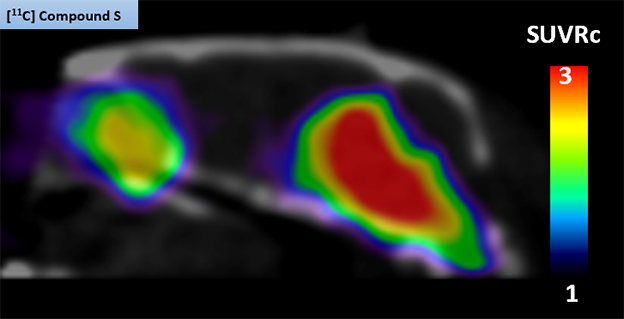
Mouse PET. The 11C-MK-7337 tracer shone brightly in the midbrains of A30P transgenic mice, but not in wild-type mice (not shown). [Courtesy of Idriss Bennacef, Merck.]
Alas, the tracer did display some off-target binding in the cerebella of rhesus macaques, raising uncertainties how it will perform in people, Bennacef said. Ongoing clinical studies aim to find out, he said.—Jessica Shugart
References
News Citations
- In First for the Field, α-Synuclein PET. Only for Multiple System Atrophy
- Behold the First Human α-Synuclein CryoEM Fibril Structure
Paper Citations
- Hsieh CJ, Ferrie JJ, Xu K, Lee I, Graham TJ, Tu Z, Yu J, Dhavale D, Kotzbauer P, Petersson EJ, Mach RH. Alpha Synuclein Fibrils Contain Multiple Binding Sites for Small Molecules. ACS Chem Neurosci. 2018 May 16; PubMed.
Other Citations
Further Reading
Papers
- Ferrie JJ, Lengyel-Zhand Z, Janssen B, Lougee MG, Giannakoulias S, Hsieh CJ, Pagar VV, Weng CC, Xu H, Graham TJ, Lee VM, Mach RH, Petersson EJ. Identification of a nanomolar affinity α-synuclein fibril imaging probe by ultra-high throughput in silico screening. Chem Sci. 2020 Dec 21;11(47):12746-12754. Epub 2020 Sep 10 PubMed.
Primary Papers
- Xiang J, Tao Y, Xia Y, Luo S, Zhao Q, Li B, Zhang X, Sun Y, Xia W, Zhang M, Kang SS, Ahn EH, Liu X, Xie F, Guan Y, Yang JJ, Bu L, Wu S, Wang X, Cao X, Liu C, Zhang Z, Li D, Ye K. Development of an α-synuclein positron emission tomography tracer for imaging synucleinopathies. Cell. 2023 Aug 3;186(16):3350-3367.e19. Epub 2023 Jul 7 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Michael J. Fox Foundation
The Michael J. Fox Foundation has supported α-synuclein PET tracer development for over a decade and has funded more than $30 million in research. It is likely that a tracer will need to be highly selective with minimal binding to other targets including other pathological proteins (Aβbeta, tau, TDP43) and bind to α-synuclein with very high affinity, likely low or subnanomolar. F0502B has many promising properties but it needs further optimization before it is ready for human testing. The affinity may need to be improved and it is unclear about potential binding to other targets. Also, it seems that the brain penetrance will need to be improved (see time-activity curves in Figure 7).
Along with the report in 2022 by AC Immune that its α-synuclein tracer, ACI-12589, shows evidence of binding to α-synuclein in the cerebellum in MSA patients, the field has finally shown progress in the development of α-synuclein PET tracers for use in research and trials. In fact, AC Immune showed data at AAIC for a newly developed tracer that shows promise for use in Parkinson’s disease studies.
In addition to the tracers mentioned above, MJFF is currently funding four projects that will test α-synuclein tracers in human subjects later this year or in early 2024. Each of these tracers bind to α-synuclein with low nM or picomolar affinity. I am aware of several other tracers that are also close to human testing. In my opinion, we are on the cusp of having a selective α-synuclein PET tracer that performs well in Parkinson’s disease.
Lewy Body Dementia Association
Building on the strength of CSF seed amplification assays for α-synuclein, there are now several efforts underway to biologically define and stage Lewy body disease, including PD, PD dementia, and dementia with Lewy bodies (DLB). These efforts represent a step forward in enabling clinical trials for disease-modifying therapies for people with Lewy pathology, potentially even preventive trials, given the recent BioFINDER and PPMI data showing that synuclein is detectible years in advance of clinical diagnosis. Furthermore, the proposed addition of synuclein to the NIA-AA criteria as non-AD pathology highlights the importance of biomarkers in differential diagnosis. Both of these dynamics will be informed and strengthened by PET tracers, which will ideally provide additional data regarding the spatial distribution of Lewy pathology in the brain during life, and enable prospective studies of the evolution of Lewy pathology, both in the natural course of disease and in response to therapeutics.
Make a Comment
To make a comment you must login or register.