From Specialized to Standardized: Social-Emotional Tests for FTD
Quick Links
One person “checks out” from family, seeming reluctant to talk. Another talks incessantly, without saying much. One person seems unaware of the emotions of others; another recognizes emotions but doesn’t seem to care. The social and emotional manifestations of frontotemporal lobar degeneration (FTLD) are complex and manifold, like everything about these conditions. How could a clinician measure them objectively, much less build standardized tests? Still, researchers are trying, not least because sensitive clinical outcome measures are so sorely needed to find out whether an investigational drug worked in a clinical trial (see Part 7 of this series).
- People with FTD suffer deep socioemotional disconnection.
- This is painful for caregivers, and difficult to quantify objectively.
- In international cohorts, tests of social and emotional cognition track disease progression.
Because FTD is rare and complicated, large international consortia, particularly Europe’s GENFI and North America’s ALLFTD, have been key to devising such tests. Their researchers are validating older neuropsychological tests that can be broadly deployed across the globe. They are also creating new ways to probe neuronal disturbances responsible for the behavioral oddities that people with FTD, and their caregivers, deal with on a daily basis. In several recent papers, and at the International Conference for FTD, held virtually March 3–5, scientists described tests that tease out deficits in social and emotional cognition, linking them to malfunction in specific neural networks in the brain.
Social and emotional deficits form the core of some FTD syndromes, notably bvFTD and semantic PPA. At ICFTD, Katherine Rankin of the University of California, San Francisco, presented data validating a suite of tests that probe this kind of dysfunction. Rankin explained that socioemotional deficits in FTD stem from crumbling circuitry in two neural networks. She called the salience network “ground zero for bvFTD.” It picks up cues from the environment that relate to our health, safety, and survival, and provokes autonomic responses, such as quickened heart rate, elevated skin conductance, and dilated pupils. Because humans are profoundly social creatures, social cues are also critical to our well-being, hence salient, Rankin said. “If my boss rolls her eyes during my presentation, that could signal grave consequences for my professional future,” Rankin said.
The semantic appraisal network is also under attack in FTD. “The SAN is a superhighway that connects valence to semantics. It supports socioemotional processing in many ways,” said Rankin. Essentially, the SAN connects semantic information to the appropriate emotional response. In the eye-rolling example, the SAN would help the presenter decide whether to feel threatened by the eye roll, or perhaps to laugh instead if the eye-rolling referred to a shared joke between friends.
While it’s clear these networks are disrupted in FTD, Rankin said that testing the degree of that with neuropsychological tests is a tall order, because it’s difficult to “fake salience” in a testing scenario. One way to approximate this is by measuring autonomic, physiological responses, such as skin conductance or pulse. A slew of candidate tests do that, but Rankin emphasized that the field urgently awaits validated neuropsychological tests, which require no special equipment and can be employed across centers and cultures.
Rankin and colleagues have been validating four socioemotional tests in NACC’s FTLD module. Most rely on an informant—a caregiver or clinician—to gauge social impairment in the person with FTD. Rankin’s personal favorite is the revised self-monitoring scale (RSMS), which gauges a person’s ability to react to subtle social cues. The test asks a close relative or friend of the patient to rank his or her social awareness and responsiveness, as gauged by how true 13 statements are. For example, one is “In conversations, the subject is sensitive to even the slightest change in the facial expression of the other person he/she is conversing with.”
Previously, the researchers had reported from work with 168 people that scores on this test distinguished those with FTD from healthy controls and, perhaps more importantly, could differentiate among FTD syndromes (Toller et al., 2018). As expected, people with bvFTD performed worst, followed by those with semantic primary progressive aphasia, Rankin said. A more recent study in the ALLFTD cohort, of 475 participants including presymptomatic mutation carriers, found changes in the RSMS prior to symptom onset that worsened with disease progression (Toller et al., 2020). In a subset of participants who returned for more than one visit, more rapid annual decline on the RSMS was significantly associated with faster shrinkage of regions belonging to the SN and SAN, such as the right anterior insula, dorsal anterior cingulate, and orbitofrontal cortex.
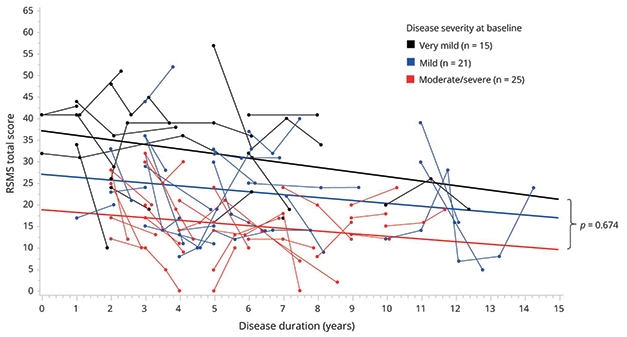
Messy but Trendy. Scores on the RSMS, a test of a person’s ability to respond to social cues, trended steadily downward as disease progressed among mutation carriers with bvFTD. [Courtesy of Toller et al., Neurology, 2020.]
The interpersonal reactivity index is another test that relies on information from a caregiver or close friend. The IRI goes a step further than the RSMS. It assesses a person’s ability to put him- or herself in another’s shoes, make an effort to understand another person’s perspective, and exhibit feelings of empathic concern when they see someone in need. Essentially, it’s a gauge of empathy. At ICFTD, Rankin reported that in the ALLFTD cohort, scores on this test tracked with disease severity as gauged by the CDR plus NACC FTLD test battery (see Part 1 of this series). For some mutation carriers, a score of zero on the CDR NACC FTLD indicated they were asymptomatic; however, their caregivers begged to differ, reporting a dearth of empathy in their partners. Similar to the RSMS, people with bvFTD had the strongest deficits on the IRI, followed by people with semantic variant of PPA. Here, too, lower scores were tied to shrinkage in key hubs of the salience and semantic appraisal networks.
Two other tests—the social behavior observer checklist (SBOCL) and the Social Norms Questionnaire (SNQ)—are also proving useful in picking up deterioration of the salience and semantic appraisal networks in ALLFTD, Rankin reported at ICFTD. Rankin and colleagues developed the SBOCL more than a decade ago to help clinicians distinguish between people with bvFTD and those with psychiatric disorders. The SBOCL is completed by a clinician, rather than a close friend or caregiver of the person with FTD (Rankin et al., 2008). After spending time with a patient, a clinician responds to questions such as “Was [the subject] overly disclosing or inappropriately familiar?” The SNQ determines the degree to which a person understands implicit but widely accepted social boundaries in American culture. For this test, the participant answers questions such as, “Would it be socially acceptable to laugh when someone else trips and falls?” Together with the RSMS and IRI, these tests of social cognition are part of the NACC-FTLD module and Gefen et al., 2020). Rankin and colleagues are about to publish a study that weaves in data from more than 1,300 people who were evaluated using this module in the NACC database. At ICFTD, Rankin reported that all four measures distinguished between people with different forms of FTD.
Most of these tests need informants. Rankin and colleagues are also devising tests that require only people with FTD, or presymptomatic mutation carriers, themselves. For example, the Dynamic Affect Recognition Test (DART) designed at UCSF plays a video of someone talking on the phone to participants, and asks them to gauge the speaker’s emotions by looking at his or her facial expressions, which change over the course of the video. The researchers are currently testing a tablet version in healthy volunteers.
These tests are not just a hodgepodge of quirky tests that work only at one center or another. The FTD field is now equipped with a growing toolkit of socioemotional measures that have been validated in large international cohorts, Rankin said. Even so, more are still needed, especially tests that work across languages and cultures. She called for investigators to keep publishing and validating neuropsych tests for FTD, even as they develop their own specialized tests to address their favorite question at their own center.
Tests of social cognition are also moving up the ranks of GENFI, the multicenter observational study of familial FTD with centers in Europe and Canada. Jason Warren of University College London heads the brain-behavior group there. Warren wants to understand how behavioral symptoms that patients and their families report to their neurologists relate to brain changes. “We want to deconstruct these symptoms into their building blocks, and find out where the mischief is in the brain,” he said. Over the past few years, Warren’s group has devised a slew of tests that use stimuli such as sound, sarcasm, humor, or body awareness, and physiological responses like pupil dilation or heart rate, to identify specific deficits in people with FTD and to zero in on their neurological bases (Nov 2014 conference news).
They have begun to roll out these tests within the larger GENFI cohort. One example is a modified version of the Camel and Cactus test, a measure of semantic knowledge. It asks participants to match pictures or words based on semantic relationships, i.e. “camel” goes with “cactus,” not “tree,” “sunflower,” or “rose.” The researchers adapted the test for use in multiple languages and cultures. Among 664 GENFI participants, symptomatic carriers of C9ORF72, GRN, or MAPT mutations performed worse than noncarriers, and deficits even emerged among presymptomatic carriers of C9ORF72 or MAPT mutations (Moore et al., 2020). The semantic deficits tracked with different atrophy patterns among the different groups of mutation carriers, correlating with atrophy in the temporal lobe in MAPT carriers, in both the temporal and frontal lobes in C9ORF72 carriers, and with shrinkage in the left frontal lobe in GRN carriers.
Two other tests helped GENFI detect early social and emotional deficits among mutation carriers. In one, participants were shown faces and asked to name the emotions on them. In the other, they were shown a series of short cartoon stories, and asked to identify those that contained a social inconvenience, or faux pas (Russell et al., 2020). Compared to controls, symptomatic people with familial FTD fared much worse on both tests. Even presymptomatic deficits cropped up in C9ORF72 carriers, who had difficulty recognizing fear and sadness, but not other emotions or faux pas. Importantly, poor performance on these tests correlated with atrophy in regions of the brain known to facilitate social cognition.
A new study in GENFI’s Dutch cohort reached similar conclusions. It used a more sensitive gauge of emotion recognition that asked participants to name emotions on faces showing different intensities of emotion (Jiskoot et al., 2021). In this computer-based test, most people more accurately named an emotion as its intensity increased. However, 32 people with Alzheimer’s disease and 32 with bvFTD scored lower across most emotions displayed than did 47 presymptomatic FTD mutation carriers and 49 healthy controls. People with bvFTD performed markedly worse than those with AD in deciphering anger and happiness. Presymptomatic mutation carriers mostly performed at the level of controls, except for C9ORF72 carriers, who had a hard time identifying mild disgust.
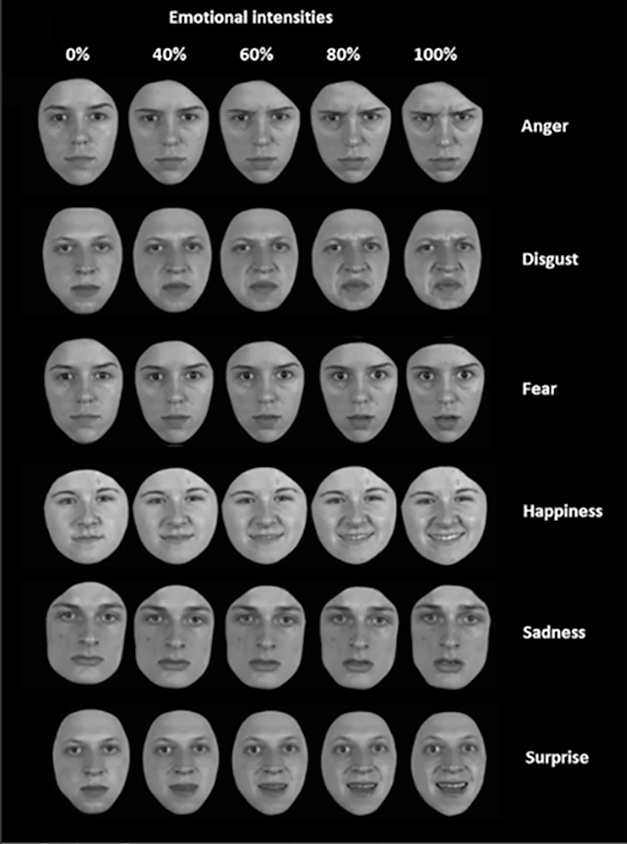
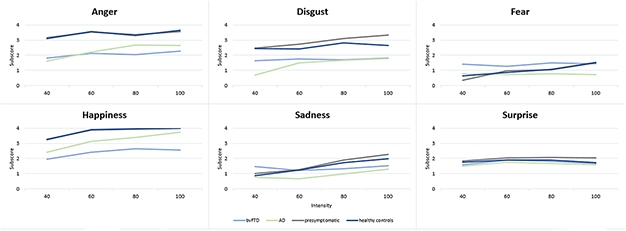
Name the Emotion. Shown images of faces displaying different intensities of one of six emotions, people with bvFTD and people with AD had a hard time labeling the emotion, especially those at low intensity. People with bvFTD scored low, especially on anger, happiness, disgust. [Courtesy of Jiskoot et al., Journal of Neurology, 2021.]
For his part, Warren is exploring better ways to tease out emotional problems among the London cohort of FTD families. For example, he noted that traditional tests of facial emotion recognition rely on complex instructions and require high levels of working memory on the part of the patient, which can confound results. To avoid this, he tracked people’s gazes to gauge emotional recognition (Russell et al., 2021). Participants were given no instructions except to observe images. As they looked at faces or, in a more difficult version of the task, sets of eyes displaying different emotions, a scanner tracked the participants’ own eye movements. Later, when shown a word fitting one of the emotions, i.e., “happy,” controls tended to rest their eyes on the face that matched that emotion, but people with bvFTD rarely zeroed in on any particular face, suggesting they did not recognize the emotion. Worse performance in the bvFTD group correlated with atrophy in the right ventromedial prefrontal and orbitofrontal cortices, brain regions previously implicated in social cognition.

Eye of the Beholder. Healthy controls spent more time looking at faces (top) or sets of eyes (bottom) that matched the displayed emotion. People with bvFTD did not focus on any one face. The color bar reflects time spent gazing at a particular area. [Courtesy of Russell et al., Alzheimer’s Research & Therapy, 2021.]
At ICFTD, Fiona Kumfor of the University of Sydney reported that people with bvFTD focus on different regions of a person’s face. Also using eye tracking, Kumfor and colleagues found that, when presented with a face showing an emotion, people with bvFTD spent more time focused on the eyes than did healthy controls. They titled their paper “Looking but not seeing” (Hutchings et al., 2018). This was counterintuitive, Kumfor said, as people with other behavioral disorders, such as autism, tend to avoid looking at eyes. Alas, their extended focus on eyes did not help people with bvFTD recognize emotions. Kumfor’s previous studies had also used physiological measures such as skin conductance and smiling/frowning measurements to reveal that people with bvFTD do not experience emotional scenes in the same way healthy people do (Kumfor et al., 2019).
That people with bvFTD would home in on eyes dovetails with findings from what Kumfor and colleagues call “a truly social approach” to measuring social cognition, i.e., just watching people converse (Visser et al., 2020). The investigators observed a 10-minute video of participants chatting with a clinician. They took note of socially engaged behaviors such as nods, smiles, and gestures, and disengaged ones, like avoiding eye contact, self-grooming, or interrupting. Compared to 20 participants with semantic dementia and 20 with Alzheimer’s, 20 people with bvFTD tended to nod less but also look away less. In fact, they tended to stare for long periods of time, a behavior that people find unsettling in social situations, Kumfor said. People with SD, on the other hand, gestured more often while talking, perhaps reflecting their reliance on nonverbal communication.—Jessica Shugart
References
News Citations
- Merged Consortia Forge Path to Trials in Frontotemporal Dementia
- Too Hot, Too Cold, or Just Wrong? Physiology Links Behavior to Circuits in FTD
Paper Citations
- Toller G, Brown J, Sollberger M, Shdo SM, Bouvet L, Sukhanov P, Seeley WW, Miller BL, Rankin KP. Individual differences in socioemotional sensitivity are an index of salience network function. Cortex. 2018 Jun;103:211-223. Epub 2018 Feb 27 PubMed.
- Toller G, Ranasinghe K, Cobigo Y, Staffaroni A, Appleby B, Brushaber D, Coppola G, Dickerson B, Domoto-Reilly K, Fields J, Fong J, Forsberg L, Ghoshal N, Graff-Radford N, Grossman M, Heuer H, Hsiung GY, Huey E, Irwin D, Kantarci K, Kaufer D, Kerwin D, Knopman D, Kornak J, Kramer J, Litvan I, Mackenzie I, Mendez M, Miller B, Rademakers R, Ramos E, Rascovsky K, Roberson E, Syrjanen J, Tartaglia C, Weintraub S, Boeve B, Boxer A, Rosen H, Rankin K, ARTFL/LEFFTDS Consortium. Revised Self-Monitoring Scale: A potential endpoint for frontotemporal dementia clinical trials. Neurology. 2020 Jun 2;94(22):e2384-e2395. Epub 2020 May 5 PubMed.
- Rankin KP, Santos-Modesitt W, Kramer JH, Pavlic D, Beckman V, Miller BL. Spontaneous social behaviors discriminate behavioral dementias from psychiatric disorders and other dementias. J Clin Psychiatry. 2008 Jan;69(1):60-73. PubMed.
- Gefen T, Teylan MA, Besser L, Pollner E, Moshkovich A, Weintraub S. Measurement and characterization of distinctive clinical phenotypes using the Frontotemporal Lobar Degeneration Module (FTLD-MOD). Alzheimers Dement. 2020 Jun;16(6):918-925. Epub 2020 May 13 PubMed.
- Moore K, Convery R, Bocchetta M, Neason M, Cash DM, Greaves C, Russell LL, Clarke MT, Peakman G, van Swieten J, Jiskoot L, Moreno F, Barandiaran M, Sanchez-Valle R, Borroni B, Laforce R Jr, Doré MC, Masellis M, Tartaglia MC, Graff C, Galimberti D, Rowe JB, Finger E, Synofzik M, Karnath HO, Vandenberghe R, de Mendonça A, Maruta C, Tagliavini F, Santana I, Ducharme S, Butler C, Gerhard A, Levin J, Danek A, Otto M, Warren JD, Rohrer JD, Genetic FTD Initiative, GENFI*. A modified Camel and Cactus Test detects presymptomatic semantic impairment in genetic frontotemporal dementia within the GENFI cohort. Appl Neuropsychol Adult. 2020 Feb 5;:1-8. PubMed.
- Russell LL, Greaves CV, Bocchetta M, Nicholas J, Convery RS, Moore K, Cash DM, van Swieten J, Jiskoot L, Moreno F, Sanchez-Valle R, Borroni B, Laforce R Jr, Masellis M, Tartaglia MC, Graff C, Rotondo E, Galimberti D, Rowe JB, Finger E, Synofzik M, Vandenberghe R, de Mendonça A, Tagliavini F, Santana I, Ducharme S, Butler C, Gerhard A, Levin J, Danek A, Otto M, Warren JD, Rohrer JD, Genetic FTD Initiative, GENFI. Social cognition impairment in genetic frontotemporal dementia within the GENFI cohort. Cortex. 2020 Dec;133:384-398. Epub 2020 Sep 26 PubMed.
- Jiskoot LC, Poos JM, Vollebergh ME, Franzen S, van Hemmen J, Papma JM, van Swieten JC, Kessels RP, van den Berg E. Emotion recognition of morphed facial expressions in presymptomatic and symptomatic frontotemporal dementia, and Alzheimer's dementia. J Neurol. 2021 Jan;268(1):102-113. Epub 2020 Jul 29 PubMed.
- Russell LL, Greaves CV, Convery RS, Nicholas J, Warren JD, Kaski D, Rohrer JD. Novel instructionless eye tracking tasks identify emotion recognition deficits in frontotemporal dementia. Alzheimers Res Ther. 2021 Feb 8;13(1):39. PubMed.
- Hutchings R, Palermo R, Bruggemann J, Hodges JR, Piguet O, Kumfor F. Looking but not seeing: Increased eye fixations in behavioural-variant frontotemporal dementia. Cortex. 2018 Jun;103:71-81. Epub 2018 Feb 27 PubMed.
- Kumfor F, Hazelton JL, Rushby JA, Hodges JR, Piguet O. Facial expressiveness and physiological arousal in frontotemporal dementia: Phenotypic clinical profiles and neural correlates. Cogn Affect Behav Neurosci. 2019 Feb;19(1):197-210. PubMed.
- Visser M, Wong S, Simonetti S, Hazelton JL, Devenney E, Ahmed RM, van Langenhove T, Parker D, Burrell JR, Hodges JR, Kumfor F. Using a second-person approach to identify disease-specific profiles of social behavior in frontotemporal dementia and Alzheimer's disease. Cortex. 2020 Dec;133:236-246. Epub 2020 Oct 6 PubMed.
External Citations
Further Reading
Papers
- Chen KH, Hua AY, Lwi SJ, Haase CM, Rosen HJ, Miller BL, Levenson RW. Smaller Volume in Left-Lateralized Brain Structures Correlates with Greater Experience of Negative Non-target Emotions in Neurodegenerative Diseases. Cereb Cortex. 2021 Jan 1;31(1):15-31. PubMed.
- Convery RS, Bocchetta M, Greaves CV, Moore KM, Cash DM, Van Swieten J, Moreno F, Sánchez-Valle R, Borroni B, Laforce R Jr, Masellis M, Tartaglia MC, Graff C, Galimberti D, Rowe JB, Finger E, Synofzik M, Vandenberghe R, de Mendonca A, Tagliavini F, Santana I, Ducharme S, Butler C, Gerhard A, Levin J, Danek A, Otto M, Warren JD, Rohrer JD, Genetic FTD Initiative (GENFI). Abnormal pain perception is associated with thalamo-cortico-striatal atrophy in C9orf72 expansion carriers in the GENFI cohort. J Neurol Neurosurg Psychiatry. 2020 Dec;91(12):1325-1328. Epub 2020 Aug 5 PubMed.
- Requena-Komuro MC, Marshall CR, Bond RL, Russell LL, Greaves C, Moore KM, Agustus JL, Benhamou E, Sivasathiaseelan H, Hardy CJ, Rohrer JD, Warren JD. Altered Time Awareness in Dementia. Front Neurol. 2020;11:291. Epub 2020 Apr 21 PubMed.
- Jimenez DA, Bond RL, Requena-Komuro MC, Sivasathiaseelan H, Marshall CR, Russell LL, Greaves C, Moore KM, Woollacott IO, Shafei R, Hardy CJ, Rohrer JD, Warren JD. Altered phobic reactions in frontotemporal dementia: A behavioural and neuroanatomical analysis. Cortex. 2020 Sep;130:100-110. Epub 2020 Jun 14 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.