Next-Generation Mouse Models: Tau Knock-ins and Human Chimeras
Quick Links
Scientists seeking better mouse models for Alzheimer’s disease recently developed human mutant APP knock-ins (see related SfN story). The slowly developing pathology in these mice may better resemble what happens in human brain, where the disease takes decades to manifest, than overexpression models do, noted Takaomi Saido of RIKEN Brain Science Institute, Wako, Japan, who generated the mice. However, for researchers trying to publish quickly, the mild phenotype and scant behavioral defects can be a disadvantage. What’s the answer?
Perhaps tau. APP knock-ins, like most APP overexpression models, lack tau neuropathology and neurodegeneration, defining aspects of human AD. The mice appear to model early, amyloid-only stages of AD. Is this due to the mice’s lifespans, or could it be due to differences between human and mouse tau? At a symposium at the Society for Neuroscience annual meeting, held November 12-16 in San Diego, Takashi Saito from Saido’s group debuted a mouse with wild-type human tau knocked in. In preliminary data, crossing this mouse with the APP knock-ins resulted in hyperphosphorylated, insoluble tau and neuronal death, Saito reported. He emphasized that the hTau knock-ins are available to academic and industry scientists who use the APP knock-in models under the same material transfer agreement. The Japanese group asked those interested to contact saido@brain.riken.jp. Another researcher at the symposium presented a cross of the APP knock-ins with transgenic mice overexpressing human tau; this combination boosted hippocampal atrophy. Such crosses could better model later stages of AD, researchers suggested.
Meanwhile, Bart De Strooper of KU Leuven, Belgium, described another approach to simulate Alzheimer’s disease. Because knock-in mice still have mouse neurons, which behave differently than human cells, his group added human induced pluripotent stem cells to transgenic mouse brain to study how amyloid pathology affects human neurons in the context of a multicellular brain environment (see below).
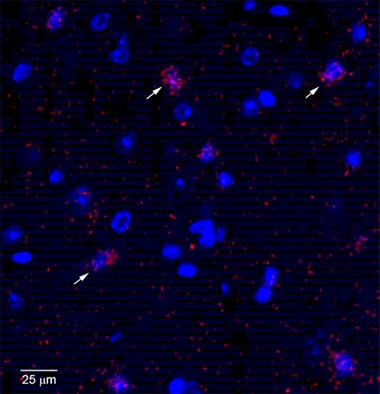
Trigger Neuronal Death.
Neurons (nuclei blue) in the entorhinal cortex of 18-month-old hTau/APPNL-F mice activate caspase-3 (red), indicating apoptosis. [Courtesy of Takaomi Saido and Takashi Saito.]
How Does Tau Fit In?
Researchers at the symposium complained about how slow research with the knock-in models is. Would adding pathological tau jack up the phenotypes? Jennifer Macdonald, working with Michel Goedert at MRC Laboratory of Molecular Biology, Cambridge, England, crossed heterozygous P301S tau mice with APPNL-F mice. At 18 months of age, the offspring had lost more hippocampal volume than their P301S parents. APPNL-F mice, by contrast, maintain normal-sized hippocampi even at two years old. The data suggest a synergistic effect of Aβ and tau on neurodegeneration.
Aβ and tau pathology converged in the subiculum of the P301S/ APPNL-F mice. There, Macdonald saw dramatic changes in microglial cells. In wild-type mice, most microglia have the normal ramified shape and only 13 percent are dystrophic with degenerating processes, whereas in P301S and APPNL-F parent mice, one-third are dystrophic. In the APPNL-F/P301S crosses, more than half the microglia were dystrophic. Rod-shaped microglia, which are associated with neurological infections and traumatic brain injury (see Bachstetter et al., 2015), make up 2 percent of the total in APPNL-F mice, 6 percent in P301S mice, and almost 10 percent in the APPNL-F/P301S crosses. Furthermore, the crosses had twice as many activated microglia, as seen by Iba1 staining, as their parents did.
The pronounced increase in dystrophic and rod-shaped microglia is similar to what is seen in AD brain, Macdonald noted. “The cross recapitulates at least two main features seen in AD: hippocampal atrophy, and microglial changes,” she wrote to Alzforum.
The overexpression problem applies to tau transgenic mice, too. The P301S mice overexpress human mutant tau, but most cases of Alzheimer’s involve normal levels of wild-type tau. To investigate the effects of normal human tau, Saito generated tau knock-in mice by humanizing the mouse tau gene. People express six tau isoforms, whereas wild-type mice express only three. The knock-in mice produce all six, Saito reported in San Diego. The animals appeared healthy and made normal amounts of tau that stayed in its customary axonal location.
Saito then crossed the hTau knock-ins with APPNL-F mice. The offspring accumulated more phosphorylated and insoluble tau than their hTau knock-in parents. Compared to the APPNL-F knock-ins, inflammatory cytokines and activated microglia were similar, but more neurons were dying by 18 months. Regions of neuronal death did not correlate with the pattern of amyloid deposition, as is also the case in AD. The hTau/APPNL-F mice activated more caspase-3 in the entorhinal cortex at 18 months (see image above). Notably, however, no neurofibrillary tangles had appeared by this age. Analysis is ongoing, with some preliminary data suggesting the entorhinal cortices of these crosses start to shrink at 21 months. The fact that the entorhinal cortex degenerates in the absence of tangles hints that oligomeric tau might be the culprit, Saido speculated.
A Chimeric Approach: Human Neurons in Mouse Brain
While the knock-in mice with humanized Aβ and tau may more faithfully model AD, they still do so with mouse neurons. Do those behave differently than human ones? De Strooper took a radically different stab at modeling AD, which allows him to ask how human neurons are affected by amyloid pathology in the complex environment of the brain. He implanted human induced pluripotent stem cells from healthy controls into the brains of newborn APP/PS1 mice, and examined brain pathology two, four, six, and eight months later.
In the 165 mice investigated to date, De Strooper and colleagues have found consistent results. Human-derived grafts develop similar levels of inflammation as surrounding mouse brain tissue, but slightly fewer amyloid plaques. Tau in the grafts becomes hyperphosphorylated, but does not form neurofibrillary tangles. However, grafts display more presynaptic pathology than host tissue, and massive neuronal death. De Strooper noted that the cell death appears necrotic, rather than apoptotic. When human cells are transplanted into wild-type mice, or mouse neurons are transplanted into the APP/PS1s, the transplanted neurons stay alive. The results suggest a unique vulnerability of human neurons to amyloid pathology, which does not depend on the presence of tau tangles, De Strooper noted. He is now analyzing gene expression changes in the grafted neurons and surrounding cells to glean clues as to why this happens.—Madolyn Bowman Rogers
References
News Citations
Research Models Citations
Paper Citations
- Bachstetter AD, Van Eldik LJ, Schmitt FA, Neltner JH, Ighodaro ET, Webster SJ, Patel E, Abner EL, Kryscio RJ, Nelson PT. Disease-related microglia heterogeneity in the hippocampus of Alzheimer's disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol Commun. 2015 May 23;3:32. PubMed.
Other Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.