Mirror, Mirror on the Wall, Who’s the Earliest of Them All?
Quick Links
At last month's Alzheimer’s Association International Conference, scientists showed new data that confirms phospho-tau231 as one of the earliest biomarkers known to rise in the plasma of people with preclinical Alzheimer’s disease, but is it really the first? According to new data also presented there, glial fibrillary acidic protein and YKL-40, two markers of astrogliosis, may rise even sooner. The findings imply that neuroinflammation begins decades before symptoms, and that astroglial markers could help identify people at risk and track their response to therapies.
- CSF GFAP and YKL40 rise as Aβ42 falls.
- These neuroinflammatory markers may change even before p-tau231.
- GFAP in the plasma, but not CSF, correlates with plaques.
“The take-home message was that you can track ‘mediators’ of Aβ toxicity using astrocytic and microglial biomarkers,” said Henrik Zetterberg, University of Gothenburg, Sweden. “This should be helpful in therapeutic trials not focusing on Aβ.”
Alas, GFAP tells a confusing story. At AAIC, scientists confirmed that levels in the plasma, but not in the CSF, correlate with amyloid plaque load, leaving them searching for an explanation.
GFAP and YKL-40 are predominantly markers of astrogliosis. Scientists have long known they tick up in AD but exactly when that starts is not clear. Yi Ting (Tina) Wang, working in the lab of Pedro Rosa-Neto at McGill University, Montreal, wanted to chart how neuroinflammatory markers behave across the AD continuum, and how they compare to markers of amyloid, tau, and neurodegeneration. Wang used PET to measure binding of the amyloid ligand AZD4694 in 210 volunteers in the TRIAD primary care cohort established by Rosa-Neto. Then, she used PET standard uptake value ratios (SUVRs) for each person to approximately pinpoint how far along they are in the disease continuum. Then using the SUVRs as a proxy for progression, she charted when GFAP, YKL-40, and other markers in the CSF begin to change. Hers was a cross-sectional analysis. “The proxy analysis gives you an idea of what the progression might look like, but we need longitudinal data to confirm,” Wang told Alzforum.
What did this first look reveal? For one, Wang noticed that CSF GFAP and YKL-40 were high, low, then high again in what appeared to be a biphasic fashion in people on different points along the progression to AD dementia. She believes the first wave indicates a stage where cells are responding to the beginning of amyloidosis; the second, Wang thinks, may reflect ineffective clearance of plaques followed by the emergence of tau pathology. The first wave would be in keeping with astrocytosis discovered very early in AD by PET (Feb 2012 news).
How do these waves resonate with other markers? Setting SUVR of 1.5 as a cutoff for amyloid positivity, Wang found that GFAP and YKL-40 began to rise very early in people who were amyloid-negative (see image below). In fact, the increase in GFAP and YKL-40 coincided with change in the CSF Aβ42/40 ratio, which falls before PET scans turn positive. Phospho-tau231 rose steeply soon after that, as did, to a lesser extent, p-tau181 and p-tau217. The order fits with data published earlier this year by Rosa-Neto and Zetterberg’s group, but see below on p-tau231 findings (Feb 2021 news).

Making Waves. Compared to amyloid PET SUVR, a proxy for disease progression, GFAP and YKL 40 rise very early in the AD continuum, fall, then rise again as plaques (top) and tangles (bottom) accumulate. The neuroinflammation markers rise earlier and reach higher concentrations in the CSF than all other markers bar NfL. [Courtesy of Tina Wang, McGill University.]
Correlating CSF and imaging markers pair-wise with each other suggested that astrocytes may indeed be reacting to waves of amyloid and tangles. In 95 volunteers without plaques or tangles, i.e., who were A-/T- in the ATN classification of amyloid, tau, and neurodegeneration markers, CSF GFAP and YKL40 did not correlate with Aβ42 and only moderately correlated with Aβ40 and p-tau181. In 23 A+/T- volunteers, i.e., people with plaques but no tangles, the inflammation markers moderately to strongly correlated with Aβ42 and with total tau. And in 62 A+/T+ participants, CSF YKL40, but not CSF GFAP, correlated with tau PET and p-tau181.
Two waves mean that trying to use these inflammatory markers in individual patients will require great care. “We need to know how to interpret the data by looking at correlations with Aβ and tau, because neuroinflammation can be caused by many other conditions,” said Wang.
Alternatively, scientists may just turn to plasma GFAP. In her talk, Wang did not discuss how this marker behaves, but her abstract does not show the same biphasic pattern (see image at left).
Others have seen the same puzzling divergence, namely that plasma GFAP correlates more tightly with AD pathology than does CSF GFAP. At ADPD last March, Andrea Benedet, in the labs of Zetterberg and Kaj Blennow at UGothenburg, reported this for 171 volunteers in the TRIAD cohort (Mar 2021 news). The revelation that a plasma solute might outperform its alter ego in the CSF took the field by surprise; after all, doesn't the former drain from the latter? Indeed, CSF markers have previously shown stronger correlations with brain pathology than their plasma counterparts.
Benedet and colleagues, working with Marc Suárez-Calvet at the Barcelonaβeta Brain Research Center in Spain have since confirmed their TRIAD findings in the ALFA+ study cohort in Spain and the BioCogBank Paris Lariboisière cohort in France. Their paper is in press.
Meanwhile at AAIC, Joana Pereira, from Stockholm's Karolinska Institutet, confirmed that plasma outdid CSF in evaluating GFAP 504 volunteers in the Swedish BioFinder cohort. Collaborating with Oskar Hansson’s group at Lund University, Pereira found more GFAP in plasma of people who tested positive for amyloid as per their CSF Aβ42/40 ratio, than in those who were negative, regardless of cognitive status. The rise in plasma GFAP seemed tied to Aβ pathology because plasma GFAP was normal even among 75 volunteers diagnosed with a non-AD dementia, such as frontotemporal, dementia with Lewy bodies, Parkinson’s, and progressive supranuclear palsy. CSF GFAP, on the other hand, rose in this non-AD group, but was unable to distinguish amyloid-positive from amyloid-negative people (see image below).
“We’ve had GFAP assays for many years, but now that we can dichotomize people by amyloid in early disease stages, we can see its power,” noted Nicholas Ashton, UGothenburg.
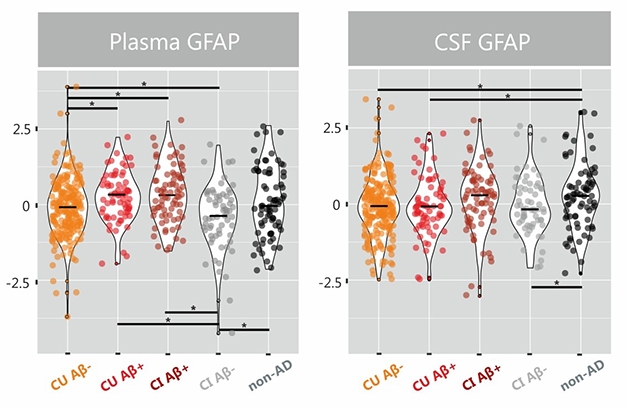
Blood Takes It. In BioFinder, plasma GFAP levels rise in Aβ-positive groups. CSF GFAP ticks higher in non-AD dementias. [Courtesy of Pereira et al., Brain 2021.]
Delving deeper, Pereira found that plasma GFAP correlated with amyloid PET not only in AD cases, but in all cognitively unimpaired individuals and groups. This fits with Wang’s data suggesting GFAP is an early marker of neuroinflammation in AD. In contrast, CSF GFAP only correlated with amyloid PET in people who were also amyloid-positive as per CSF, and only when they were also cognitively impaired. When calculated voxel by voxel, only plasma GFAP correlated with amyloid, once again suggesting a more sensitive correlation.
As in Wang’s cohort, Pereira, too, found that plasma GFAP tracked Aβ PET SUVRs. When plotting sensitivity over specificity, plasma GFAP predicted PET positivity with an AUC of 0.76, versus 0.69 for CSF GFAP. Both markers predicted cognitive decline in longitudinal analyses, though only plasma GFAP predicted amyloid accumulation.
All these studies used the same Quanterix SIMOA assay for GFAP. This eliminates errors arising from assay or platform variations, said Ashton. “Unlike for p-tau species, there is only the one GFAP assay, so it is really developing consistent result across cohorts,” he said.
Even so, scientists are perplexed by the contrast between plasma and CSF GFAP. The former seems to reflect amyloid accumulation, whereas the latter may reflect neuroinflammation caused by other pathologies. “It is really a surprise to everyone,” said Ashton. But why does only plasma GFAP predict amyloid? “It is counterintuitive,” agreed Michelle Mielke, Mayo Clinic, Rochester, Minnesota. “I have not heard a good reason for it.” Ashton told Alzforum that scientists are focusing on two potential explanations. One is that GFAP degrades faster in the CSF, and may be more sensitive to freeze/thaw cycles, which could skew data from stored samples. Ashton favors this idea, and would like to test if there is a difference between fresh plasma and fresh CSF. Or perhaps GFAP does not enter plasma via CSF, but through some other mechanism, possibly directly from astrocytes into the blood vessels. This idea is plausible because astrocytes are in direct contact with pericytes on small blood vessels of the neurovascular unit.
Indeed, both Ashton and Mielke noted that plasma GFAP might shoot up in cerebrovascular disorders and traumatic brain injury. “We need a comprehensive study that looks at both cerebrovascular and AD pathology, aging, atrophy, and various cognitive domains to fully understand exactly what this assay is measuring and how it may be more useful,” said Mielke. “That said, there are studies that suggest it is more specific to AD neurodegeneration, so that is really promising.”
As for p-tau, that other early marker, Ashton presented the latest at AAIC. Earlier this year, he had seen that plasma p-tau231 was one of the earliest of them all, appearing before p-tau181 and before PET scans could detect amyloid. That data came from four different cohorts, including a small autopsy group of 47 brain donors (Feb 2021 news). Working with Douglas Galasko, University of California, San Diego, Ashton has since added a second autopsy cohort of 312 patients at UCSD’s Alzheimer’s Disease Research Center. These volunteers had had plasma samples taken every other year for decades, including last samples taken within five years of death, and reflect a more heterogenous population.
Similar to the prior data, plasma p-tau231 distinguished AD from other neurodegenerative diseases and strongly correlated with CERAD scores of neuritic plaques and with Braak stage at autopsy. In people who had died at Braak stage V/VI, plasma p-tau231 had risen in plasma at least 10 years prior. Further, plasma p-tau231 best predicted cognitive decline over four years. Similarly, in samples from PREVENT-AD, a Canadian study that tracks cognitively normal people at risk for AD, Ashton found that in those who tested positive for amyloid based on plasma Aβ42/40 levels, plasma p-tau231 predicted subtle cognitive decline over six years.
How about in people with early signs of AD pathogenesis? For this, Ashton turned to the ALFA+ cohort in Spain. Among 384 people, average age 61, who are cognitively healthy and had PET scans for amyloid, plasma p-tau231 predicted Aβ-positivity better than did plasma neurofilament light or plasma p-tau181, but no better than plasma GFAP. However, all tau markers outperformed plasma GFAP in correlating with change in CSF Aβ42/40.
Could this be where plasma p-tau231 shines? “We think this is a robust clinical biomarker that outperforms plasma GFAP and p-tau181 in predicting soluble Aβ changes that occur prior to plaque formation,” said Ashton.—Tom Fagan
References
News Citations
Further Reading
Papers
- Chatterjee P, Pedrini S, Stoops E, Goozee K, Villemagne VL, Asih PR, Verberk IM, Dave P, Taddei K, Sohrabi HR, Zetterberg H, Blennow K, Teunissen CE, Vanderstichele HM, Martins RN. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer's disease. Transl Psychiatry. 2021 Jan 11;11(1):27. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
PolygenicPathways
The tau gene MAPT contains five cryptic peptides (cryptides) with antimicrobial properties against bacteria and fungi (Kobayashi et al., 2008). One of these, VQIVYK, is contained within the sequence PGGGS_ VQIVYK_PVDLSK (Microtubule Tau Binding Region, MTBR tau 299) that is upregulated in the AD brain and in CSF in early stages of the disease, and is reported to be one of the earliest biomarkers (Horie et al., 2021).
References:
Kobayashi N, Masuda J, Kudoh J, Shimizu N, Yoshida T. Binding sites on tau proteins as components for antimicrobial peptides. Biocontrol Sci. 2008 Jun;13(2):49-56. PubMed.
Horie K, Barthélemy NR, Sato C, Bateman RJ. CSF tau microtubule binding region identifies tau tangle and clinical stages of Alzheimer's disease. Brain. 2021 Mar 3;144(2):515-527. PubMed. Correction.…More
Make a Comment
To make a comment you must login or register.