At the Heart of Alzheimer’s in Down’s: Cerebrovascular Disease
Quick Links
Almost everyone who has Down’s syndrome (DS) is fated to develop Alzheimer’s disease because they have three copies of the amyloid precursor protein (APP) gene. Their cells overproduce Aβ, they become amyloid- and tau-positive on PET by age 40, and they develop dementia by age 55, on average. This would make them candidates for anti-amyloid immunotherapies, yet they have been excluded from most studies on these treatments thus far. Why? Safety is a concern. Many people with DS have severe cerebral amyloid angiopathy (CAA), which scientists suspect contributes to ARIA, an inflammation of the brain that can be provoked by immunotherapy (see Part 6 of this series).
- People with Down’s syndrome have healthy hearts and peripheral blood vessels.
- And yet they have cerebrovascular disease.
- Cerebral amyloid angiopathy may be to blame.
- Understanding CAA in DS is important for immunotherapy.
“Focusing on cerebrovascular pathology in DS is critically important for considering clinical trials with the newly approved immunotherapies against Aβ,” Elizabeth Head, University of California, Irvine, wrote to Alzforum.
Figuring out how and when CAA and cerebrovascular disease begin, and how they progress throughout life, are important goals for the Down’s field. At the Alzheimer’s Association International Conference last month in Amsterdam, scientists reported that signs appear in a person’s 30s and worsen with age. Further, people with more markers of cerebrovascular disease had worse amyloid and tangle pathology, suggesting the vascular pathology marches in lockstep with AD.
“To me, this means cerebrovascular disease is not simply a comorbidity—it is an important part of DSAD,” Adam Brickman of New York’s Columbia University told Alzforum.
Cerebrovascular and Alzheimer's Disease Pathology
Brickman and Alexandre Bejanin at the Hospital de Sant Pau, Barcelona, separately found the simultaneous rise in markers of cerebrovascular disease and AD by analyzing brain scans and fluid biomarkers from people enrolled in two cohorts: the NIH-funded Alzheimer Biomarker Consortium‐Down Syndrome (ABC-DS), which has eight U.S. study sites and one at the U.K.’s University of Cambridge, and the Down Alzheimer Barcelona Neuroimaging Initiative (DABNI) cohort based at Hospital de Sant Pau. Brickman assessed around 240 ABC-DS participants with different analyses, while Bejanin studied up to 250 from DABNI.
Patrick Lao from Brickman’s lab reported that people with DS become amyloid-positive, as judged by PET, at around age 35, and tangle-positive at age 39. Signs of vascular injury begin to appear on MRI scans around then too. Spaces around blood vessels enlarged. Infarcts, a marker of large-vessel disease, emerged in the early 30s. Microbleeds, signs of CAA, and white-matter hyperintensities (WMHs), signs of small-vessel disease, ticked up around age 36 (see image below).

Terrible 30s. People with DS begin accumulating amyloid plaques around age 35 and neurofibrillary tangles at 39 (not shown). Perivascular swelling (top left) and infarcts (top right) appear around age 31. Microbleeds (bottom left) and white-matter hyperintensities (bottom right) start to worsen at 36 and 37, respectively. [Courtesy of Adam Brickman and Patrick Lao, Columbia University.]
In a similar vein, Sára Zsadányi reported a rise in microbleed prevalence and severity around age 40, while Alejandra Morcillo-Nieto found WMHs flare up around the same time. Both work in Bejanin’s lab. In volunteers with microbleeds, hippocampi had begun to shrink while CSFAβ42/40 ratios began to fall and phospho-tau181 to climb, both signs of accumulating plaques. CSF neurofilament light had ticked up also, indicating neuronal damage.
Natalie Edwards in Brickman’s lab saw similar fluid marker changes in plasma. People with many WMHs had high p-tau217, NfL, and plasma glial fibrillary acidic protein (GFAP) compared to controls. These data indicate that vascular injuries worsen as AD pathology progresses.
By the time people with DS develop AD dementia in their 50s, their cerebrovascular disease has progressed so much that their blood-brain barrier became compromised, said Lisi Flores Aguilar from Head’s lab at UC Irvine. She reported that DS occipital cortex tissue was flooded with the blood protein fibrinogen, which barely entered the brain parenchyma of age-matched controls. Fibrinogen only leaks into the brain when vessels are damaged, and its presence in parenchyma indicates BBB breakdown. All told, the evidence supports Brickman’s contention that cerebrovascular disease is a core feature of DSAD and worsens in step with neuropathology.
Cardiovascular Risk in DS
Is cerebrovascular disease in Down’s primarily driven by cardiovascular disease, a major risk factor for late-onset AD? No. People with DS generally do not have heart disease or high blood pressure, according to Sarah Pape of King’s College London. Pape analyzed primary care records from 6,500 people with DS ages 18 to 75 and 23,000 age-matched controls. Half as many DS adults had heart disease or high cholesterol as did controls, and only one-quarter as many, or fewer than 1 percent of the DS population, developed high blood pressure. While 99 percent of people with DS will never have high blood pressure, Brickman and Bejanin’s work show that many will develop microbleeds and WMHs. “Vascular risk factors may exacerbate cerebrovascular disease, but they don’t seem necessary for it to emerge in DS,” Brickman concluded.
Those who did have hypertension were twice as likely to develop dementia by age 55. “People with DS who have cardiovascular disease are at increased risk of AD, just like people who do not have DS,” Andre Strydom, King’s College London, who chaired the DS session, told Alzforum.
When Plaques Meet the Cerebrovasculature
The poor correlation between cardiovascular and cerebrovascular disease in DS supports the idea that CAA causes the latter. Researchers know that severe buildup of Aβ in blood vessel walls weakens them enough to burst. Indeed, microbleeds, a sign of this damage, correlate with CAA in postmortem cortical tissue from DS cases beginning in the mid-30s (Helman et al., 2019). This mirrors the rise in amyloid plaques seen on brain scans starting around that age.

CAA in Dup-APP. Compared to arteries from a person who had Down’s (top), those from a person with the Dup-APP mutation (bottom) had even more amyloid (brown). [Courtesy of Marie-Claude Potier, Paris Brain Institute.]
But is amyloid alone sufficient to cause CAA in DS? To gain a more nuanced understanding, Marie-Claude Potier of the Paris Brain Institute compared postmortem cortical tissue from people who had had DSAD and those who had a rare duplication of APP but no DS. Dup-APP causes early onset AD and CAA (Rovelet-Lecrux et al., 2006). Potier found that while DSAD arteries and vessels had lots of CAA, those from people with the duplication had even more. CAA in the Dup-APP cases was so severe, even their brain capillaries had it, a rarity in other forms of AD (see image at right).
What explains the difference? While more Aβ was found in Dup-APP brains than in DS brains, the isoforms also differed. Shorter Aβ peptides—including Aβ34, Aβ38, Aβ40, and their N-truncated varieties—were more abundant in Dup-APP tissue, though the amount of Aβ42 was similar. In collaboration with Jörg Hanrieder at the University of Gothenburg, Potier used imaging mass spec to find that the short and N-truncated Aβ peptides are exclusively embedded in blood vessel walls of Dup-APP cases, while Aβ42 preferentially aggregated in the parenchyma (see image below). These data suggest that the overabundance of short Aβ peptides in people with the duplication, and in DS to a lesser extent, causes CAA. This mirrors where isoforms home to in CAA among the general population (Alonzo et al., 1998).
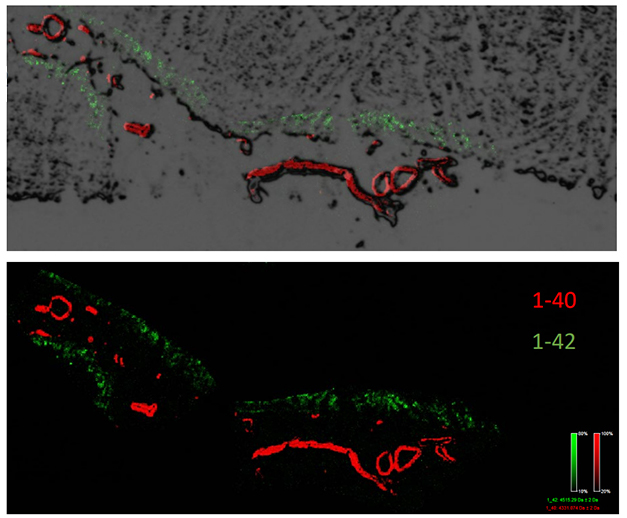
Short Peptides Flock to Vessels. While Aβ42 (green) aggregated in the parenchyma of Dup-APP tissue, Aβ40 (red) preferentially settled in the blood vessels. So did Aβ39, Aβ38, and their N-truncated forms (not pictured). [Courtesy of Marie-Claude Potier, Paris Brain Institute.]
Implications for Immunotherapy
As a strong risk factor for ARIA, a side effect of anti-amyloid antibodies, CAA might pose an added hurdle for using immunotherapy to help people with DS fend off Alzheimer's dementia. “It raises a cautionary note, given that … people with DS are more vulnerable to cerebrovascular pathology than are people with late-onset AD,” wrote Head. At the same time, demand for treatment in this population will rise as these drugs become more widely available.
Knowing that CAA and cerebrovascular disease both worsen in the mid-30s will help researchers decide when to start people with DS on these anti-amyloid drugs to avoid ARIA as much as possible. “We may want to start a very low dose or start young, when cerebrovascular problems are not fully developed,” Head added. In a consensus statement on amyloid immunotherapy in DS, Head and colleagues recommended including people with Down’s syndrome in clinical trials, and developing protocols to help monitor such drug usage.
No plans to evaluate any anti-amyloid antibody in DS have been announced. “There are ongoing discussions as we consider how best to safely bring these amyloid-lowering immunotherapies to the DS population,” Michael Rafii, University of Southern California, told Alzforum. The one immunotherapy that is being tested in people with DS is the anti-amyloid vaccine ACI-24 (May 2021 news).
To gear up for trials, researchers are looking to find better markers of pathology and better cognitive tests for DS. This has proven challenging given the broad range of intellectual ability accompanying DS. Head, Brickman, and Donna Wilcock of Indiana University in Indianapolis are collaborating to study longitudinal changes in cerebrovascular pathology in DS in search of fluid markers of cerebrovascular disease. This may help screen people at increased risk of CAA and, potentially, ARIA. A similar search for markers of CAA is ongoing in sporadic AD (Part 7 of this series).
Jason Hassenstab at WashU plans to develop a remote cognitive assessment designed for DS—the first of its kind. His will be a smartphone app consisting of short, gamified tasks that measure memory, attention, and speed. People will “play” them multiple times a day for a week until they’ve completed all the tests. Hassenstab plans to launch the app in 2025.—Chelsea Weidman Burke
References
News Citations
- Is ARIA an Inflammatory Reaction to Vascular Amyloid?
- In Down's Syndrome, Amyloid Vaccine Opens Door to Trials
- Wanted: Fluid Biomarkers for CAA, ARIA
Mutations Citations
Mutation Position Table Citations
Therapeutics Citations
Paper Citations
- Helman AM, Siever M, McCarty KL, Lott IT, Doran E, Abner EL, Schmitt FA, Head E. Microbleeds and Cerebral Amyloid Angiopathy in the Brains of People with Down Syndrome with Alzheimer's Disease. J Alzheimers Dis. 2019;67(1):103-112. PubMed.
- Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerrière A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006 Jan;38(1):24-6. Epub 2005 Dec 20 PubMed.
- Alonzo NC, Hyman BT, Rebeck GW, Greenberg SM. Progression of cerebral amyloid angiopathy: accumulation of amyloid-beta40 in affected vessels. J Neuropathol Exp Neurol. 1998 Apr;57(4):353-9. PubMed.
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
Hospital of Sant Pau
The DS and cerebrovascular disease sessions at AAIC showed that both DSAD and ADAD have more CAA than sporadic AD, and it has been suggested that individuals with DSAD have fewer hemorrhages than individuals with APP duplication. In this sense the studies comparing the underlying processes in the three forms of AD offer unique perspectives.
With respect to anti-amyloid antibodies, the immediate question is whether people with DS will be allowed to receive them. We all share the concern about safety because people with DS have not been exposed to these drugs and, as mentioned, have more CAA and are potentially at higher risk.
On the other hand, some of us are also worried about equitable access. No medication used in DS has been specifically tested in DS, e.g., COVID vaccines, insulin, etc., despite different immune responses and so on. People with ADAD, who share the increased CAA, will be able to receive these antibodies; they, of course, have been exposed to gantenerumab and crenezumab. It is a very difficult conundrum in my view. We are fighting hard to have a safety trial. We hope this be possible soon.…More
Of note, there is near unanimity in the field that DSAD, because of the numbers, might be the best population in which to conduct clinical trials.
Make a Comment
To make a comment you must login or register.