In Head-to-Head Testing, P-Tau217/Tau217 Comes Out on Top. By a Hair.
Quick Links
With many different blood markers and assays for helping doctors diagnose Alzheimer’s disease, which are they to choose? Head-to-head studies can help answer this question, but few have directly compared blood tests on the same samples.
Last year at CTAD, Nick Ashton, then at University of Gothenburg, Sweden, presented data from a small round-robin that compared the performance of 26 different p-tau tests on 40 blood samples taken from people suspected of having AD. p-Tau217 came out on top (Nov 2023 conference news). Most of the tests were immunoassays.
Not represented was the p-tau217/tau217 ratio. The percentage of fragments phosphorylated at this residue is emerging as being perhaps a more robust marker than the absolute level of p-tau217. Now, two new head-to-head studies, one led by Suzanne Schindler of Washington University, St. Louis, the other by Oskar Hansson and Noëlle Warmenhoven at Sweden’s Lund University, support the idea that %p-tau217 is a more accurate marker, though the differences between tests are often marginal. The studies are described in manuscripts uploaded to medRxiv early in July and were recently presented at AAIC in Philadelphia.
Warmenhoven and colleagues compared mass spectrometry-based assays for %p-tau217 and p-tau217 run at Washington University with commercial immunoassays for p-tau217 from Lilly, Janssen, and ALZpath. They used plasma samples from 998 volunteers in the Swedish BioFinder-2 cohort, of whom 375 were cognitively healthy, the remainder having subjective cognitive impairment, MCI, AD, or dementia due to another cause. Almost all had had PET scans for neurofibrillary tangles; 694 had had amyloid PET scans.
The %p-tau217 test proved a tad more accurate in identifying people who were amyloid-positive. The p-tau217 mass spec test performed similarly to the Lilly and ALZpath p-tau217 immunoassays, and all three edged out the Janssen test. Most assays posted accuracies in the high 80s for identifying samples from tangle-positive volunteers, with the exception of p-tau181 (image below).
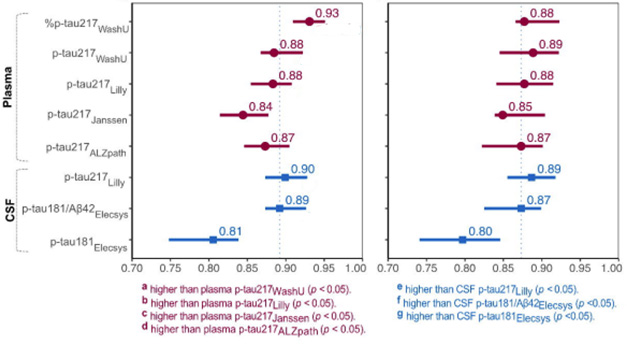
Testing, Testing. Plasma tests (purple) for p-tau217 identified amyloid-positive (left) and tangle-positive (right) people in BioFinder-2 with comparable accuracy. They are as good, if not better, than CSF tests (blue), including the FDA-approved Elecsys test for p-tau181/Aβ42 from Roche (dashed vertical line for reference). [Courtesy of Warmenhoven et al., 2024.]
Furthermore, the scientists compared how well each test correlated with amyloid and tangle load, as well as with presence of both pathologies, baseline cognitive scores, and decline from baseline. On all measures, the %p-tau217 correlated as well, or slightly better, than did the other tests, with Lilly’s immunoassay generally being the strongest of the immunoassays. Warmenhoven found a similar pattern in a smaller cohort of 219 volunteers from the Knight Alzheimer’s Disease Research Center at WashU.
The authors concluded that the %p-tau217 test might be considered as a stand-alone confirmatory test for AD, while the immunoassays might be better suited to triage. WashU’s Randall Bateman, who co-founded C2N Diagnostics, St. Louis, was a co-author on the paper. C2N sells PrecivityAD2, a mass spec-based test for AD that measures %p-tau217 and the Aβ42/40 ratio (Dec 2023 news).
Going Head-to-Head in ADNI
For her part, Schindlers’s medRxiv manuscript reports on an analysis commissioned by the Biomarker Consortium of the Foundation for the NIH. In a prior study, the consortium had compared six assays measuring plasma Aβ42/40 (Zicha et al., 2022). For the current one, they added the analytes %p-tau217, p-tau217, p-tau181, the glial marker GFAP, and the neurodegeneration marker NFL.
This was the biggest head-to-head study yet. It pitted these assays against each other: C2N’s Aβ42, Aβ40, p-tau217, and tau217; Fujirebio Diagnostics’ Lumipulse tests for p-tau217 and Aβ42 and Aβ40; ALZpath’s Quanterix p-tau217; Janssen’s LucentAD Quanterix p-tau217; Roche Diagnostics’ NeuroToolKit assays for p-tau181, Aβ42/Aβ40, GFAP, and NfL; and Quanterix’s Neurology 4-Plex tests for p-tau181, Aβ42/Aβ40, GFAP, and NfL. Most of these tests are commercially available.
At AAIC, first author Kellen Petersen, WashU, reported that the scientists ran each of these tests on aliquots of 392 samples from the ADNI cohort. They first appraised each test for its ability to detect people with brain amyloid. The authors then correlated each test with each participant’s tau PET status, cortical atrophy, and cognitive impairment.
Here, too, the %p-tau217, as measured by C2N, generally outperformed the immunoassays. It identified amyloid-positive people with an accuracy of 0.87. The ALZpath and Fujirebio p-tau217 assays came in a close second, with accuracies of 0.84 and 0.83, respectively, followed by Janssen’s 0.82. All did much better at detecting brain amyloid than did tests of p-tau181, GFAP, or NfL (table below).
The same pattern emerged when correlating the tests with the burden of amyloid, not just positivity as defined by a specific cutoff. Including the Aβ42/40 ratio where available, i.e., the C2N and Fujirebio platforms, did not significantly improve performance.

P-tau217. Again. Among 392 individuals tested in the ADNI cohort, plasma %p-tau217 most accurately identified who had brain amyloid. Immunoassays for p-tau217 came in a close second. [Courtesy of Schindler et al., 2024.]
In general, the tests did better in plasma from a subgroup of those 192 volunteers who were cognitively impaired, i.e., people who would qualify for biomarker testing in clinical practice under current guidelines. Here, adding the plasma Aβ42/40 data did improve things slightly, with the Fujirebio and C2N tests both posting accuracies of 90 percent. “This means that when trying to determine if someone who is cognitively impaired has Alzheimer’s, the immunoassays will work similarly to mass-spec assays,” said Schindler. In prevention studies, or a clinical trial that enrolls cognitively unimpaired people, mass spec tests may perform better.
Curiously, including plasma Aβ42/40 boosted accuracies most in people who were amyloid-negative on PET. “It seems the plasma Aβ42/40 ratio helps very early on in the disease trajectory, when there is a low level of amyloid,” said Schindler. Then as p-tau217 begins to rise and the change in Aβ42/40 plateaus, the latter becomes less informative.
For all the other outcome measures—tau PET, cortical thickness, and dementia severity—correlations were strongest with the %p-tau217 and p-tau217 tests (image below). “This is notable because, right now, we have different markers for ‘ATN’, or amyloid, tau, and neurodegeneration,” said Schindler (see Nov 2023 conference news on diagnostic criteria). “Why would you use Aβ42/40, or NfL, when p-tau217 is better?”

One Size Fits All. Rather than having separate markers for “ATN” the amyloid, tau, and neurodegeneration of AD, could just p-tau217 do the job? In ADNI, it best correlated with amyloid, tangles, atrophy, and cognitive impairment. [Courtesy of Schindler et al., 2024.]
Adieu Lumbar Punctures?
What does all this mean for CSF testing? In Schindler’s view, given how well some of the plasma tests perform, it is getting harder to justify lumbar punctures to collect CSF. “The major reason clinicians still do it is because blood tests are not reimbursed,” she told Alzforum.
C2N and Fujirebio have submitted applications to the FDA; others may soon follow. Given the current momentum in the AD field, some scientists at AAIC were hopeful that approval for plasma tests might come as early as next year. “In the not-too-distant future, we will be doing blood tests on most people,” Schindler said. Others at AAIC shared this view.—Tom Fagan
References
News Citations
- Plasma p-Tau-217 Assays Work Well, But No Home Run for Diagnosis
- Two New p-Tau217 Blood Tests Join a Crowded Field
- New Alzheimer’s Diagnostic Criteria Remain ‘Research Only’
Paper Citations
- Zicha S, Bateman RJ, Shaw LM, Zetterberg H, Bannon AW, Horton WA, Baratta M, Kolb HC, Dobler I, Mordashova Y, Saad ZS, Raunig DL, Spanakis EM, Li Y, Schindler SE, Ferber K, Rubel CE, Martone RL, Weber CJ, Edelmayer RM, Meyers EA, Bollinger JG, Rosenbaugh EG, Potter WZ, Alzheimer's Disease Neuroimaging Initiative (ADNI), Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium Plasma Aβ as a Predictor of Amyloid Positivity in Alzheimer's Disease Project Team. Comparative analytical performance of multiple plasma Aβ42 and Aβ40 assays and their ability to predict positron emission tomography amyloid positivity. Alzheimers Dement. 2022 Jul 12; PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Schindler SE, Petersen KK, Saef B, Tosun D, Shaw LM, Zetterberg H, Dage JL, Ferber K, Triana-Baltzer G, Du-Cuny L, Li Y, Coomaraswamy J, Baratta M, Mordashova Y, Saad ZS, Raunig DL, Ashton NJ, Meyers EA, Rubel CE, Rosenbaugh EG, Bannon AW, Potter WZ. Head-to-head comparison of leading blood tests for Alzheimer's disease pathology. 2024 Jul 03 10.1101/2024.06.12.24308839 (version 2) medRxiv.
- Warmenhoven N, Salvado G, Janelidze S, Mattsson-Carlgren N, Bali D, OrdunaDolado A, Kolb H, Triana-Baltzer G, Barthelemy NR, Schindler SE, Aschenbrenner AJ, Raji CA, Benzinger TL, Morris JC, Ibanez L, Timsina J, Cruchaga C, Bateman RJ, Ashton NJ, Arslan B, Zetterberg H, Blennow K, PichetBinette A, Hansson O. A Comprehensive Head-to-Head Comparison of Key Plasma Phosphorylated Tau 217 Biomarker Tests. 2024 Jul 05 10.1101/2024.07.02.24309629 (version 1) medRxiv.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.