Emerging Alzheimer’s Therapies Test the Waters at CTAD
Quick Links
Beyond keynote speeches and eagerly awaited immunotherapy data, many presentations at the Clinical Trials on Alzheimer’s Disease (CTAD) conference, held December 8-10 in San Diego, detailed findings from therapies in the earliest stages of development. These were tested in small groups of participants, some without placebo controls. Here are a few highlights from trial tidbits reported at the conference.
Philip Scheltens of VU University Medical Center in Amsterdam presented data from a Phase 2a trial of VX-745, aka Neflamapimod. This p38MAPK inhibitor is proposed to reduce harmful inflammation, boost microglial phagocytosis, and improve neuronal plasticity. Sixteen people in Europe with MCI or mild AD received either 40 mg or 125 mg of VX-745 twice daily for 12 weeks. Fifteen (eight in the 40 mg group and seven in the 125 mg group) completed the trial and were evaluated at the VU Medical Center. The drug was well-tolerated and caused no severe adverse events, Scheltens said.
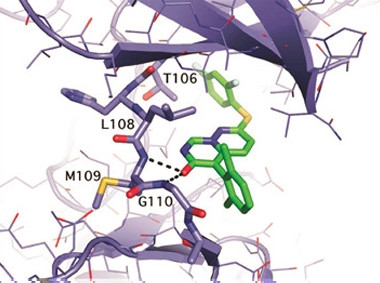
Kinase Blocker.
Crystal structure of VX-745 (green) bound to p38 MAPKα (purple). [Courtesy of John Alam.]
Researchers evaluated the primary outcome measure—reduction in Aβ deposition—via quantitative PET imaging. This method, which continuously measures PiB in the brain throughout a 90-minute scan, is more sensitive and less prone to variability than standard amyloid-PET scans, which take a briefer measurement at a specific time, usually 60 to 90 minutes after the tracer has been injected into the blood stream. Because of the tighter variability of this PET method, the researchers decided ahead of time that an amyloid reduction of more than 7 percent would constitute a bona fide response to the drug, while a 3 percent to 7 percent reduction would be considered a partial response. They found that in the 40 mg group, three people had a full response, two had a partial response, and three did not respond. In the 125 mg group, only one out of the seven had a full response. Interestingly, that person had a plasma level of the drug on par with people in the 40 mg group. All four full responders had plasma VX-745 exposure below 90 nghr/ml.
Scheltens reported that the four full responders also had had the lowest baseline levels of amyloid deposition. He concluded that the lower, 40 mg dose was better for reduction of amyloid, and that three months of treatment was perhaps insufficient to reduce amyloid deposition in those who started out with high baseline levels. Scheltens could not explain these paradoxical dose findings, although he offered that it is possible that the higher dose may go too far in shutting down inflammatory responses needed to mop up Aβ.
In a secondary outcome measure, the Wechsler Memory Scale test, participants in both dose groups improved throughout the trial. However, Scheltens acknowledged that because this small trial had no placebo group, it would be difficult to rule out that practice effects were at play.
Separately, John Alam of EIP Pharma in Cambridge, Massachusetts, reviewed results from a U.S.-based CSF analysis study with the same basic design, though it was shorter—six weeks rather than 12. Sixteen people with MCI or mild AD were slated to receive either 40 mg or 125 mg of VX-745 twice daily. However, after the trial began, the FDA introduced a limit on dosing, to keep plasma VX-745 levels tenfold below the level causing toxicity in long-term animal studies. Because the drug sponsors expected plasma VX-745 might exceed that limit in people taking the high dose, they suspended that arm after only three people had been enrolled and one had completed the study. Alam told Alzforum that because the European Union interprets the toxicity differently, the high-dose arm could be completed in Amsterdam.
The U.S. study measured memory using the Hopkins Verbal Learning Test–Revised (HVLT-R), a test that affords little improvement with practice. This was evident by minimal improvement of most participants in two tests before they took their first dose, Alam said. In the HVLT-R, participants are asked to remember as many words as possible from a list of 12, either immediately or 20 to 25 minutes after hearing them. Seven and five of the eight participants on 40 mg VX-745 improved significantly in the immediate and delayed recall scores, respectively, at the end of the trial.
Alam reported analysis of CSF taken from the eight participants who received the 40 mg dose of VX-745 and the one who completed the 125 mg dose regimen before that was stopped. Since that person’s plasma VX-745 was on par with those in the 40 mg dose, the researchers pooled the data. Because VX-745 is thought to tamp down neuroinflammation, the researchers attempted to measure the CSF concentrations of nine cytokines, but ultimately only one—IL-8—was consistently detectable. CSF levels of this pro-inflammatory cytokine significantly dropped in three participants who had the highest plasma levels of VX-745, all above 90 nghr/ml, suggesting that the drug does have some anti-inflammatory effects, Alam reported. These same three participants with less CSF IL-8 also had significantly elevated CSF levels of Aβ38 and Aβ40 relative to their baselines, with a similar trend for Aβ42
While the PET and CSF analysis were undertaken in different patients, at different centers, Alam told Alzforum that it does not appear VX-745 reduces amyloid plaque via an anti-inflammatory effect (i.e., by reducing IL-8 levels). The PET responders in Amsterdam all had plasma drug exposures below 90 nghr/ml, while those in the United States whose plasma IL-8 fell all had drug exposure above that level. Alam said the data is in keeping with preclinical studies suggesting VX-745 has interleukin signaling effects mediated by inhibition of p38α, and interleukin production effects mediated by inhibition of p38β. The drug’s IC50 for the former is less than for the latter, Alam said. This may explain why lower doses of the drug might preferentially reduce Aβ—higher doses could interfere with its clearance. Suppressing p38α may attenuate BACE cleavage of APP, and slow production of Aβ42 (see Schnöder et al., 2016). Alam thinks this may be how the drug improves cognition, because that might reduce Aβ toxicity at the synapse. The researchers hope to further test the drug’s effects on Aβ burden and cognitive improvement in a larger, placebo-controlled trial, Scheltens said.
Ruolun Qiu of Pfizer in Cambridge, Massachusetts laid out data from PF-06648671, a small molecule designed to clamp down on γ-secretase’s production of amyloidogenic peptides from APP, while sparing processing of other crucial substrates, such as Notch 1. Qiu presented safety and CSF Aβ data from single- and multiple-dose studies.
In a single-dose study, 22 healthy participants, ages 18 to 55, received one oral dose of 150 mg or 300 mg of PF-0664871, or a placebo, and their CSF was monitored continually for 36 hours via a lumbar cannula. Aβ40 and 42 levels reached a nadir at 16 hours—dropping by an average of 23 percent and 39 percent, respectively, in response to the 300 mg dose. In a multiple-ascending-dose study on healthy volunteers in the same age group, six cohorts of 10 (eight active, two placebo) participants received daily doses ranging from 4 mg to 360 mg of the modulator, or placebo, for 14 days. On day 14, the researchers observed a dose-dependent reduction in Aβ42—by 14, 43, 59, and 65 percent for the 40, 100, 200, and 360 mg doses, respectively. Aβ40 also dropped dose-dependently, but by lesser amounts, resulting in a reduction in the Aβ42/40 ratio just as with the single dose.
On the other hand, shorter peptides shot up in response to treatment. Aβ37 rose by 335 percent and 372 percent after 14 days on 200 mg and 360 mg doses, respectively, while Aβ38 rose by 46 percent after 14 days on the 200 mg dose. Some of these peptides are considered non-amyloidogenic, but the full range of Aβ species has not been exhaustively studied. Despite a dramatic shift in the length of certain Aβ peptides, treatment did not affect the concentration of total Aβ, Qiu said. These findings suggested to the researchers that the drug could limit production of toxic Aβ species while otherwise preserving γ-secretase function. Qiu reported that the drug was safe and well-tolerated at the tested dose range, and that the most common adverse events—headache, dizziness, and nausea—were likely due to the lumbar puncture procedure. The safety profile also looked good in a third study in healthy elderly people, ages 65-85, who received 200 mg doses for two weeks, Qiu said.
Another drug aimed at altering neuronal activity, AGB101, is poised to enter Phase 3. An extended release version of the anti-epileptic drug levetiracetam, AGB101 is proposed to dampen the neuronal hyperactivity in the hippocampus that occurs in people with mild cognitive impairment. In a Phase 2 study, the drug appeared to do exactly that, and people performed better on memory tests (see Mar 2015 news). At CTAD, Richard Mohs of AgeneBio in Baltimore and colleagues reported that following a positive meeting with the FDA, the company will move forward with Phase 3 trials to test the drug’s effects on cognition. Starting in mid-2017, they will randomize 830 people with MCI due to AD to receive placebo or 220 mg of AGB101 for 78 weeks. The primary outcome measure will be improvement on the clinical dementia rating scale sum of boxes (CDR-SB). Participants will also undergo other cognitive tests and structural MRI to track the drug’s effects on neurodegeneration. AgeneBio has spent 2016 preparing and raising money for this Phase 3 study (see slide deck).
A Placebo-Free Space for Therapy Trials?
Tackling AD from a metabolic angle, John Didsbury of T3D Therapeutics in Research Triangle Park, North Carolina, presented findings from a Phase 2a study of T3D-959 in patients with mild to moderate AD. The hope is that this small-molecule agonist of PPAR delta/gamma nuclear receptors will improve waning metabolism in the AD brain by increasing insulin sensitivity. Thirty-six participants were randomized to receive 3, 10, 30, or 90 mg daily oral doses of the drug for 14 days. There was no placebo group.
On treatment day 14, FDG-PET imaging indicated dose-dependent changes compared to baseline in cerebral glucose metabolism in brain regions typically affected by AD. For the most part, the study measured regional to whole-brain ratios of glucose metabolism, which went down in a dose-dependent manner. To Didsbury, this indicated that the drug penetrated the brain and engaged its target.
On a secondary outcome measure, the ADAS-Cog11, 17 of the 32 participants available for testing at day 14 reportedly improved by at about one point over baseline, while 12 and nine participants improved by two or three points, respectively. Improvements held steady when participants were re-tested one week later. Despite the small number of participants on whom to base his conclusions, Didsbury claimed the dose response differed by ApoE4 genotype. Among non-carriers, those in the lower-dose groups improved over the course of the study, while those in the highest-dose group got worse. Carriers, on the other hand, had a more classic dose response, improving more at higher doses, said Didsbury. He said that researchers will take ApoE4 status into account in future, placebo-controlled studies. Others have noted that two- to three-week cognitive data, much less genetic stratification, in small, short trials, without a placebo group, carry little meaning.
A combination of repurposed drugs also was reported at CTAD, when researchers from Pharnext in Paris presented data from a single-blind, exploratory Phase 2 trial of PXT-864. In keeping with the company’s modus operandi of putting previously approved drugs to new use, this regimen consists of the GABA-B receptor agonist baclophen (a muscle relaxant) and the small molecule acamprosate (approved to treat alcohol dependence). Pharnext researchers propose that the cocktail could help balance inhibitory and excitatory synaptic signals skewed by Aβ oligomers. The trial started as a 12-week pilot study called PLEODIAL I. Forty-five people with mild AD received one of three combination doses twice daily (0.4 mg acamprosate and 6 mg baclofen, 1 mg acamprosate and 15 mg baclofen, or 20 mg acamprosate and 12 mg baclofen). There was no placebo group. Participants took the combination for two four-week stints, with a four-week placebo break in the middle. Then, in a 24-week extension study called PLEODIALII, 39 volunteers continued the treatment and had the option of taking 5 mg donepezil along with PXT-864 during the last 12 weeks of the extension.
At CTAD, René Goedkoop of Pharnext reported that over the course of the 36-week trial, participants in each dose group declined more slowly—by 0.6 to 0.9 points—on the ADAS-Cog11 than did published, historical placebo groups. However, in other contemporary treatment trials, too, placebo groups sometimes decline more slowly than historical controls. When the researchers removed participants who took donepezil during the last 12 weeks of the trial from the analysis, they found that people in the lowest-dose group actually declined faster than historical placebos—by an average of 2.3 points— over the course of the trial. Removing donepezil-takers did not affect the results in mid- or high-dose groups, but the findings were confounded by an uneven distribution of donepezil takers: Seven of 13 participants on the low dose, compared to only two of 14 in the middle and one of 13 on the high dose, opted to take donepezil.
On a poster, Karim Bennys of the University Hospital in Montpellier, France, presented results of event-related potential (ERP) recordings of three participants in each dose group of the PXT-864 trial. The latency and amplitude of ERPs in response to a task are thought to reflect working memory or decision-making. Both measures shot up during the treatment phases relative to baseline and the interim placebo phase, in people on the low and middle doses. To Bennys, this indicated that the drug may have a neurophysiological effect. At 36 weeks, the three people on the low dose had the largest improvements in ERP latency and amplitude; however, all three of them also took donepezil during the last 12 weeks of the trial (those on mid and high doses did not take donepezil). After the first four weeks of the trial, the six participants on the highest combination dose had improved in ERP latency and amplitudes, however, they declined following the next treatment phase.
Goedkoop told Alzforum that it is difficult to say whether this group performed more poorly due to the absolute dose, or to the different ratio of acamprosate to baclofen in that group, which was 5:3 rather than 1:15. The researchers aim to tease out these dose effects, and the interaction with donepezil, in future double-blind, placebo-controlled Phase 2 trials, Goedkoop said.—Jessica Shugart
References
Therapeutics Citations
News Citations
Paper Citations
- Schnöder L, Hao W, Qin Y, Liu S, Tomic I, Liu X, Fassbender K, Liu Y. Deficiency of Neuronal p38α MAPK Attenuates Amyloid Pathology in Alzheimer Disease Mouse and Cell Models through Facilitating Lysosomal Degradation of BACE1. J Biol Chem. 2016 Jan 29;291(5):2067-79. Epub 2015 Dec 9 PubMed.
Other Citations
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.