Donanemab: Small Tweak in Titration, Big Gain in Safety?
Quick Links
Seeking ways to improve the safety of anti-amyloid antibodies, John Sims of Eli Lilly & Company presented a simple option to do so for donanemab at this year’s Clinical Trials on Alzheimer’s Disease conference, held October 29-November 1 in Madrid. Starting at a low dose, and stepping it up over the first four months, cut the rate of ARIA-E by 40 percent, generating fewer severe cases to boot, Sims told the CTAD audience. The difference was biggest for APOE4 homozygotes, whose risk dropped by two-thirds, almost to the level in heterozygotes. Efficacy at clearing amyloid remained the same. Sims said Lilly will submit the data to global regulators to consider updating the label.
- When donanemab was titrated up in steps, ARIA-E risk dropped.
- For APOE4 homozygotes, risk fell to nearly the level in heterozygotes.
- New donanemab AUR are stricter than the label, emphasizing safety.
Gil Rabinovici of the University of California, San Francisco, called the data exciting. Also in Madrid, Rabinovici presented newly minted appropriate use recommendations for donanemab. They are similar to those for aducanumab and lecanemab, except that they call for more rigorous MRI criteria to exclude patients at higher risk for ARIA. A paper detailing the AUR is currently under review.
Rabinovici noted the manuscript might be updated to include Lilly’s modified titration protocol. Meanwhile, UCSF is already instituting the new titration scheme Sims presented at CTAD, he told Alzforum.
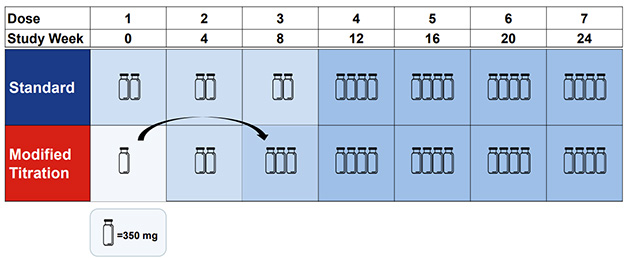
Move Over, Vial. In a new titration protocol (bottom), one vial of antibody moves from the first dose to the third; total donanemab exposure in the first six months remains identical to the standard protocol (top). [Courtesy of Eli Lilly.]
Simple Change, Big Payoff
In the Phase 3 Trailblazer-Alz2 trial, about a quarter of people on donanemab developed ARIA-E, with a fourth of those having symptoms such as headaches or confusion. The risk was highest in APOE4 homozygotes, with 40 percent of them experiencing edema (Jul 2023 conference news).
Lilly ran the Trailblazer-Alz6 trial to test different titration schemes in hopes of mitigating this. After all, the company is rolling out clinical use of donanemab at a time of intense scrutiny for anti-amyloid immunotherapy, and while their antibody is before regulators abroad.
Trailblazer-Alz6 enrolled 843 people, randomized to one of four dosing arms. The first tested the standard protocol, in which participants received three monthly doses of 700 mg, then 1,400 mg monthly thereafter. Arm 2 used a stepwise titration, with participants receiving doses of 350, 700, and 1,050 mg in the first three months (image above). In the third arm, participants had a first dose of 700 mg, skipped a month to let their brain acclimate, then went to 1,400. In the final arm, participants got 350 mg biweekly for the first three months, 700 mg biweekly in the fourth month, and 1,400 monthly thereafter. The goal there was to keep doses lower by spreading them out, with the theory that higher antibody concentration might provoke ARIA. In all four schemes, the cumulative donanemab exposure after four months was the same.
The scientists compared ARIA rates after six months, because in previous trials 90 percent of ARIA had occurred by then. As expected, the standard protocol produced the same amount of ARIA-E as in trials, 24 percent. Both skipping a dose, or spreading out doses, nudged down ARIA-E to about 18 percent, but this difference was not significant. The stepwise strategy, however, dropped the overall rate nearly in half, to 14 percent. For comparison, this is similar to the 12 percent ARIA-E rate reported for lecanemab.

Erasing Excess Risk. Under the standard titration protocol (blue), more than half of APOE4 homozygotes got ARIA-E; under a stepwise protocol (red), their risk resembled that of other genotypes. [Courtesy of Eli Lilly.]
For the 21 APOE4 homozygotes on the stepwise protocol, the change slashed their ARIA rate from 57 to 19 percent, near to the level in heterozygotes and noncarriers (image at right).
When ARIA did arise, it was less serious, Sims said. Three percent of people on stepwise titration had symptomatic ARIA-E, compared with 5 percent on the standard protocol. No ARIA-E cases were rated severe on MRI, compared with 2 percent severe on the standard protocol.
There were also fewer signs of the microhemorrhages known as ARIA-H. These often occur after edema; with the stepwise titration, the rate of concurrent ARIA-E and -H dropped from 16 to 10 percent. Rates of superficial siderosis, a sign of brain bleeds, halved, from 13 to 7 percent.
Despite this better safety overall, one person on the stepwise protocol died from ARIA. The person was an APOE4 carrier who had mild ARIA-E on the 24-week MRI scan, and went to the emergency room a week later with seizures and partial paralysis. There, the person was misdiagnosed as having a stroke and given the clot-buster tenecteplase, which led to a large brain bleed. Misdiagnosing ARIA-E as stroke, and giving tissue plasminogen-activator drugs, is emerging as a main cause of death for patients on amyloid immunotherapy. Most medical centers that prescribe anti-amyloid antibodies now have procedures in place to prevent this from happening. Alas, patients may show up at different ERs in case of crisis or when they travel. “Hospitals will have to learn not to accidentally kill Alzheimer’s patients on these antibodies by giving them thrombolytics,” Lawrence Honig of Columbia University, New York City, said with characteristic bluntness.
Meanwhile, the slower titration did not diminish plaque removal. The stepwise protocol cleared an average of 56 centiloids per person, versus 59 on the standard protocol, and it lowered plasma p-tau217 as much as did standard treatment. Cognition was not assessed in this trial, which is still ongoing, with participants being followed to 76 weeks, Sims noted.
The results suggest ARIA might be triggered by the initial contact between anti-amyloid antibodies and vascular plaque, Sims said. By keeping the first doses low, these reactions can be ameliorated, as the vasculature has slightly more time to adjust to incoming antibodies. “The first dose is important,” he said in Madrid.
AUR Emphasize Caution
To aid doctors who are now beginning to prescribe these antibodies, the clinician-researchers who had developed previous AURs for aducanumab and lecanemab have now issued recommendations for donanemab, as well (Aug 2021 conference news; Aug 2022 conference news; Apr 2023 conference news).
The group has expanded to 12 members. In addition to Rabinovici, they are: Paul Aisen of the University of Southern California in San Diego; Liana Apostolova of Indiana University School of Medicine in Indianapolis; Alireza Atri of Banner Sun Health Research Institute in Sun City, Arizona; Jeffrey Cummings of the University of Nevada, Las Vegas; Steven Greenberg of Massachusetts General Hospital, Boston; Suzanne Hendrix of Pentara Corporation, Salt Lake City; Ron Petersen of the Mayo Clinic in Rochester, Minnesota; Stephen Salloway of Butler Hospital in Providence, Rhode Island; Suzanne Schindler of Washington University, St. Louis; Dennis Selkoe of Brigham and Women’s Hospital, Boston; and Michael Weiner of the University of California, San Francisco.
Every anti-amyloid antibody is different, hence the donanemab AUR address two unique aspects of this drug: in its Phase 3 trial, scientists used baseline tau PET scans to determine eligibility, and they stopped dosing after a given person’s brain amyloid load had dropped below a preset threshold. For clinical practice, the AUR do not recommend tau PET. They do note that, if a scan is available, it can be used to estimate the benefit a person might experience from treatment. Regarding how long to treat, the AUR suggest clinicians consider stopping donanemab based on a visually negative amyloid PET scan taken after 12 to 18 months of infusions.
On safety, Rabinovici said the donanemab recommendations are meant primarily for physicians who have limited experience with this class of drugs. As such, the AUR are more stringent than the FDA label, erring on the side of caution. This is especially important during this sensitive initial period of learning how amyloid immunotherapy performs in the real world beyond clinical trial sites.
For example, the AUR suggest not treating patients who also take anticoagulants, because too few people in the Phase 3 trial were on these drugs to be able to assess donanemab’s risk in them. This recommendation will be subject to change as more data emerge, Rabinovici said. On the other hand, the AUR do not restrict anti-platelet drugs such as aspirin, since those were common in the trial and not associated with increased risk.
Both suggestions are in line with the lecanemab AUR. For lecanemab, there was evidence that anticoagulants amped up the risk of large brain bleeds and death in the Phase 3 trial (Jan 2023 news).
In another divergence from the label, the donanemab AUR recommend not treating patients who have even one area of superficial siderosis in their brains at baseline. These iron deposits, a relic of past bleeds, can indicate cerebral amyloid angiopathy, which amplifies the risk of ARIA. The Phase 3 trial had allowed one baseline area of superficial siderosis. However, an analysis by Greenberg linked this to a doubled risk of ARIA-E (Nov 2023 conference news). Others have found baseline siderosis in people who died on amyloid immunotherapy (Jan 2024 news; Jun 2024 news; Aug 2024 conference news). And also at CTAD in Madrid, trontinemab—the vanguard of a hopefully safer class of anti-amyloid antibodies to come—was shown to have been associated with a macrohemorrhage death likely linked to a baseline area of superficial siderosis, as well (see previous story in this series).
“We agreed unanimously that patients with baseline superficial siderosis should be excluded from treatment pending further safety data,” Rabinovici wrote to Alzforum. The lecanemab AUR have the same stricture. Moreover, Rabinovici noted that Lilly excludes people with baseline siderosis in its new trial of remternetug. “The field as a whole seems to be moving toward this position,” Rabinovici said.
In addition, if more than one area of superficial siderosis crops up during treatment, the AUR recommend stopping the drug. Some members of the AUR group thought even one occurrence was reason to stop, Rabinovici noted.
Scientists in Madrid held a spirited debate about how conservative to be on safety, and whether the FDA prescription insert or the AUR better guide clinicians. For example, both the donanemab and lecanemab AUR recommend against treating people with autoimmune disorders such as lupus, or with prior strokes. Honig has given lecanemab to patients with lupus, or who had recovered from a stroke years ago, and said they are doing well so far. He argued that because there is no clinical evidence for increased risk, the drug should not be withheld from these patients. “I believe in patient autonomy. Every patient should be offered a drug if it’s reasonable to do so, and the patient should consider the risk,” Honig said. In general, because the FDA label is more closely based on the clinical trial results than are the AUR, Honig prefers them.
Meanwhile, Salloway and others advocated for following the AUR. Salloway noted that he has heard from patients on autoimmune treatments who had severe, life-threatening ARIA. “Regulators and clinicians in some parts of the world are very concerned about safety,” he said.
What guidelines are memory clinics following? Previously, Alzforum found mixed results. Many clinics adhere strictly to the AUR. Others consider the label to be the firmer guide (Jan 2024 news). The issue is likely to stay in flux as more clinics begin using these drugs and real-world data comes in.—Madolyn Bowman Rogers
References
Therapeutics Citations
News Citations
- Donanemab Data Anchors Upbeat AAIC
- Aducanumab: Will Appropriate-Use Recommendations Speed Uptake?
- Bringing Aduhelm—and Antibodies to Come—Into Practice
- Next Goals for Immunotherapy: Make It Safer, Less of a Hassle
- Should People on Blood Thinners Forego Leqembi?
- Unlocking Blood-Brain Barrier Boosts Immunotherapy Efficacy, Lowers ARIA
- Brain of Woman Who Died on Leqembi Shows Worst-Case Scenario
- Donanemab Approval Likely to Pose New Quandaries for Clinicians
- Two New Deaths on Leqembi Highlight Need to Better Manage ARIA
- Trontinemab Data Strengthen Hope for Brain Shuttles
- Rising Leqembi Prescriptions Are Straining Clinic Capacity
Other Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.