Can Exercise Slow the Progression of Alzheimer’s Pathology?
Quick Links
Part 2 of two.
Evidence for the cognitive benefits of exercise keeps growing, but researchers are still not sure how it helps the brain. At the Alzheimer's Association International Conference 2015, held July 18-23 in Washington, D.C., several speakers presented imaging data that addressed this question. Overall, the findings indicated that working out enhances vascular brain health and connectivity, implying a direct benefit to brain structure and function. Data were mixed on whether exercise slows the progression of underlying Alzheimer’s pathology, however. One six-month study of moderate aerobic exercise reported a drop in cerebrospinal fluid tau in cognitively impaired people, but a shorter intervention failed to budge brain amyloid in people with AD. In general, speakers agreed that the cognitive boost from exercise likely comes from diverse benefits on several different aspects of brain function, something that would be hard to match pharmacologically. “There are multiple pathways for how it affects cognitive health, and that would be hard to package into a single pill,” Teresa Liu-Ambrose at the University of British Columbia, Vancouver, told Alzforum.
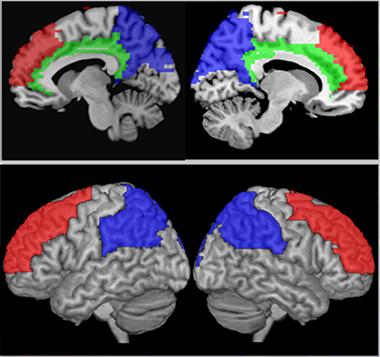
Blood Flow Boost.
In people who exercised aerobically for six months, cerebral blood flow increased in the regions most vulnerable to aging and AD (prefrontal cortex, red; posterior parietal, blue; cingulate, green). [Courtesy of Laura Baker.]
One of the biggest questions is whether exercise can slow neurodegenerative processes. Laura Baker of Wake Forest University Health Sciences, Winston-Salem, North Carolina, provided some of the first evidence for this in her talk on the PACE-2 study. This trial enrolled 65 older adults with amnestic mild cognitive impairment and high blood-sugar levels. Half of them took six months of supervised, moderate-intensity aerobic exercise classes, while the rest attended low-intensity stretching classes. Researchers collected cerebrospinal fluid biomarkers at baseline and study completion.
CSF profiles changed in exercisers. With age, levels of CSF tau and phosphorylated tau normally rise, perhaps reflecting neuron death. In trial participants over 70, CSF p-tau levels instead fell with exercise, suggesting better brain health in this vulnerable population. CSF Aβ42 also varied between exercise and control groups, although those data are harder to interpret. This marker rises during normal aging, but begins to drop once amyloid starts depositing in the brain. In controls over 70, the researchers saw Aβ42 climb over the course of the study, whereas it remained stable in exercisers. Baker suggested that these data might represent a progression of aging in controls, but not exercisers. She plans to investigate this further in future studies by adding amyloid imaging to estimate where participants might be on the trajectory of amyloid deposition.
The results contrast with findings from the Danish Phase 3 ADEX study of exercise and AD. This 16-week trial enrolled 200 people with moderate Alzheimer’s, half of whom exercised aerobically three times per week. Exercise did not seem to slow pathology in this study. A subgroup of 34 participants who volunteered for amyloid imaging continued to accumulate brain amyloid at the same rate as the control group, Kristian Steen Frederiksen at the Danish Dementia Research Centre, Copenhagen, reported in D.C. Preliminary analysis of CSF from another 37 participants likewise revealed no change in Aβ42, according to a poster presented by Camilla Steen Jensen, also at the Danish Dementia Research Centre. However, Frederiksen noted that 16 weeks may not have been long enough to effect measurable changes on biomarkers. He pointed out that animal work and observational studies both provide encouraging evidence for the potential of physical activity to move biomarkers. “There’s still a lot of hope that exercise, even at the dementia stage, might modify disease,” he told Alzforum. The researchers are still analyzing biomarker and imaging data from this trial, and will continue to follow the exercise cohort to measure long-term effects of the intervention.
The Head-Heart Connection: Better Brain Vascular Health in Exercisers
How else might exercise yield cognitive benefits? One of the most obvious effects of exercise on the brain is through cerebral blood flow. Supporting this idea, Baker and colleagues recorded enhanced blood flow in the brain in the PACE-2 exercise group, specifically in prefrontal regions, where perfusion normally drops with age, and in posterior parietal regions, where flow ebbs during Alzheimer’s disease (see image above). Other studies have reported improved glucose metabolism in those regions as a result of exercise as well, she noted. Brain glucose metabolism wanes during AD. “Moderate-intensity aerobic exercise can attenuate the effects of aging and AD on brain function,” Baker concluded.
Other data adds to this hypothesis, including a study conducted by Elizabeth Boots, Stephanie Schultz, and Ozioma Okonkwo at the Wisconsin Alzheimer's Institute in Madison. They have measured fitness, brain structure, and cognitive function in 106 cognitively healthy older adults with an average age of 64 as part of the UW Fitness, Aging, and the Brain study. The participants also take part in the Wisconsin Registry for Alzheimer’s Prevention, a longitudinal study, and many have a family history of Alzheimer’s. In D.C., Boots reported that those with the greatest cardiorespiratory capacity, as judged by peak oxygen consumption, had greater cerebral blood flow in the angular gyri and right temporal cortex, regions implicated in AD. The fittest participants also did better on several tests of executive function. Moreover, less-fit participants accumulated more white matter lesions at older ages, indicating more wear and tear on the brain. By contrast, while the youngest of the fit participants had the same number of lesions as their less-fit counterparts, the number did not go up with age in the fitter volunteers. Exercise might attenuate this aging-related decline in brain health, Schultz suggested. The researchers are now conducting a six-month randomized controlled trial of aerobic exercise in this at-risk population. “It seems likely that what’s good for your heart is good for your brain,” Schultz noted.
With Exercise, Brain Connections Snap, Crackle, and Pop
Imaging studies delineate other alterations in brain structure and function in exercisers. In D.C., Liu-Ambrose reported data from the PROMOTE study of 60 older adults with mild vascular cognitive impairment. A subset of 21 participants underwent functional MRI while performing a task that measured selective attention. Those who had exercised for six months reacted faster and with greater accuracy than the controls. They also activated fewer brain regions, suggesting more efficient cognitive performance.
Structural MRI of another 30 PROMOTE participants found an increase in white matter and a drop in gray matter in the 16 exercisers. It is unclear why gray matter volume fell, but other studies have reported a drop in gray matter paired with better cognition after various treatments (see Jul 2004 conference news; Nov 2012 conference news). The volume loss may reflect amyloid and associated fluid moving out of the brain, or dampened inflammation, Liu-Ambrose speculated. In an upcoming study, she will add further imaging measures such as amyloid PET and analyze blood biomarkers of inflammation to get a better idea of what is going on. She will also investigate whether the proliferation of white matter lesions stops in exercisers.
In a poster, Rodrigo Dennis Perea, now at Massachusetts General Hospital, Boston, focused on the brain’s structural connections. While at the University of Kansas Alzheimer’s Disease Center, Fairway, Perea had found that AD patients with high cardiovascular fitness preserved white matter integrity better than their less-fit peers (see Perea et al., 2015). The causal relationship was unclear, however. To see if exercise could improve this white-matter integrity, he scanned 30 AD patients with structural and diffusion tensor imaging (DTI) MRI. The latter traces white-matter tracts. He took images before and after half of them completed six months of aerobic exercise. Exercisers developed greater connectivity between the thalamus and right cingulate gyrus, and less connectivity between the thalamus and left post-central gyrus, compared with controls. The data indicate that exercise can modify brain structure even in people with dementia, Perea noted. The findings complement Liu-Ambrose’s data on increased white matter after exercise, and other small studies tying exercise to greater functional connectivity and more efficient use of the brain (see, e.g., Rajab et al., 2014; Wang et al., 2015).
Wait, There’s More—Genes and Growth Factors
Exercise may also help the brain by pumping up production of brain-derived neurotrophic factor (BDNF), a key compound for neurogenesis, learning, and memory. The relationship between exercise and BDNF was first found in animal studies, but has also been reported in people (see May 2002 news; Cotman and Berchtold, 2002). Moreover, BDNF levels are low in AD (see May 2009 news). While BDNF may mediate some benefits of exercise, data presented at AAIC by Carla Nascimento of the Federal University of São Carlos, Brazil, suggest it does not explain the whole picture. A cohort of 47 cognitively impaired older adults took part in either an exercise intervention or normal care for 16 weeks. Eleven of the exercisers and 10 of the control group carried the Val66Met polymorphism in BDNF, which results in less secretion of the growth factor. Only people without the Met polymorphism gained a boost in peripheral BDNF after the exercise intervention, but all exercisers did better on a test of executive function. In addition, plasma levels of two inflammatory factors, TNF-α and IL-6, dropped in exercisers regardless of BDNF genotype (see Nascimento et al., 2015). The data imply that exercise can sharpen cognition and calm inflammation through mechanisms other than BDNF.
Exercise may even modify genetic risk. A subset of 50 participants in the UW Fitness, Aging, and the Brain study donated DNA, which the researchers used to genotype the AD risk genes ApoE, clusterin, and ABCA7. All three genes are involved in cholesterol metabolism. A combined risk score from the three genes associated with lower CSFAβ42 and a higher total tau/Aβ42 ratio, indicating worse pathology, in participants with poor fitness. In their fitter peers, this genetic risk was nearly abolished, with fluid biomarkers approaching the levels seen in people with no risk genes. “We think of genetic risk as something you can’t change, but this shows there are things one can do to protect against brain changes,” Schultz told Alzforum.
Despite all these clues, exactly how exercise supports cognition remains hazy. “That’s the million-dollar question,” Baker said.—Madolyn Bowman Rogers
References
News Citations
- Philadelphia: Can a Shrinking Brain Be Good for You?
- CTAD: New Data on Sola, Bapi, Spark Theragnostics Debate
- Run For Your Brain: Exercise Boosts Hippocampal Gene Expression, Neurogenesis
- Research Brief: Low Spinal Fluid BDNF a Prelude to Memory Decline?
Paper Citations
- Perea RD, Vidoni ED, Morris JK, Graves RS, Burns JM, Honea RA. Cardiorespiratory fitness and white matter integrity in Alzheimer's disease. Brain Imaging Behav. 2015 Aug 4; PubMed.
- Rajab AS, Crane DE, Middleton LE, Robertson AD, Hampson M, MacIntosh BJ. A single session of exercise increases connectivity in sensorimotor-related brain networks: a resting-state fMRI study in young healthy adults. Front Hum Neurosci. 2014;8:625. Epub 2014 Aug 14 PubMed.
- Wang Z, Guo Y, Myers KG, Heintz R, Peng YH, Maarek JM, Holschneider DP. Exercise alters resting-state functional connectivity of motor circuits in parkinsonian rats. Neurobiol Aging. 2015 Jan;36(1):536-44. Epub 2014 Aug 16 PubMed.
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002 Jun;25(6):295-301. PubMed.
- Nascimento CM, Pereira JR, Pires de Andrade L, Garuffi M, Ayan C, Kerr DS, Talib LL, Cominetti MR, Stella F. Physical exercise improves peripheral BDNF levels and cognitive functions in mild cognitive impairment elderly with different bdnf Val66Met genotypes. J Alzheimers Dis. 2015;43(1):81-91. PubMed.
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.