Blood Tests Go Head-to-Head in Community Cohorts
Quick Links
Part 2 of 3
Only five years ago, the advent of a blood test that would root out amyloid plaques lurking in a person's brain was just emerging on the horizon (Jul 2017 news). Now, multiple such tests—particularly the Aβ42/40 ratio and several phospho-tau species—have proven to be quite exquisite detectors of amyloid plaques, at least among well-characterized research cohorts. At the Alzheimer’s Association International Conference, held July 31-August 4 in San Diego, scientists presented new findings in more heterogenous community cohorts, and compared multiple assays head-to-head. Among p-tau assays, mass spectrometry-based tests came out on top, though a handful of immunoassays were not far behind. Researchers agreed that different combinations of biomarkers may suit different clinical stages of disease, with fewer needed to detect amyloid as symptoms worsen.
- In patients with uncertain clinical diagnoses, mass spec assays for plasma Aβ42/40 and p-tau217 predict brain amyloid well.
- Direct comparisons of plasma p-tau assays reveal that several are sensitive, with mass spec in the lead.
- Plasma GFAP adds value to biomarker palette both early and late in disease.
Charlotte Teunissen of Amsterdam University Medical Center laid out a five-phase roadmap of biomarker development (Part 1). Exploration and clinical assay development comprised the first two, while in phase 3, the biomarkers are put to the test in retrospective studies using banked blood samples from longitudinal studies, including population-based cohorts that tend to be more heterogenous than typical research cohorts. At least for the best-established biomarkers thus far, many of the data shown at AAIC focused on this third phase, as scientists tried out the most promising biomarkers in community cohorts.
Blood Biomarker Palette
Tim West from C2N Diagnostics in St. Louis presented biomarker findings from 221 participants in the ongoing Plasma Test for Amyloidosis Risk Screening study. PARIS is a prospective add-on to the community-based Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) study. All participants in PARIS have cognitive symptoms; for 65 percent, their amyloid PET scan was positive. In prior research cohorts, C2N’s plasma Aβ42/40 ratio assay, a mass spectrometry test that is commercially available for order by dementia specialists, has predicted the likelihood of amyloid positivity with an area under the curve of above 0.9, West noted. AUC is a combined measure of specificity and sensitivity in which 1.0 is the highest score.
In the PARIS-IDEAS cohort, the blood test predicted amyloid-PET positivity with an AUC of 0.79, the researchers recently reported (Hu et al., 2022). Why the dip? IDEAS enrolls Medicare patients whose physicians have deemed them “difficult to diagnose,” making them candidates for amyloid PET. In contrast, most research cohorts include people who participate in longitudinal aging studies, enabling a clearer delineation of persons who are healthy from those who have MCI, or dementia due to AD. For this reason, the lower AUC of plasma Aβ42/40 in the PARIS-IDEAS came as no surprise, C2N’s Joel Braunstein told Alzforum. As in previous evaluations of this test, including a measure of age and ApoE prototype—the latter measured by mass spec—nudged up the AUC significantly, to 0.86 in this cohort. This combination of age, plasma Aβ42/40, and ApoE prototype—sold as PrecivityADTM—yields an amyloid probability score. C2N is currently marketing this combined test to dementia specialists. According to an August 23 press release, the company has joined forces with Eisai, Inc., to market blood biomarker tests in traditionally underserved communities.
A bigger improvement was to be had by adding plasma p-tau217. As many presentations at AAIC made clear, the plasma concentration of various phospho-tau species, including p-tau181, 217, and 231, are sensitive detectors of brain amyloid plaques, not of tau tangles as seen by PET. C2N has a mass spec test for p-tau217 and, at AAIC, West reported how it performed alone or in combination with Aβ42/40 in PARIS-IDEAS. On its own, plasma p-tau217 outperformed the Aβ ratio test, with an AUC of 0.92. Accuracy climbed to 0.95 when the ratio of phosphorylated to unphosphorylated tau at this residue was measured instead of the concentration p-tau217 alone. Combining this tau ratio with the Aβ42/40 ratio achieved an AUC of 0.96, although that was not significantly higher than the p-tau217/tau217 ratio alone.
“Even in a difficult-to-diagnose, real-world population of patients with MCI/dementia of unknown etiology, the combined test demonstrated outstanding performance,” Braunstein said.
Under what circumstances would clinicians need both biomarkers, if p-tau217 appears to do the job by itself? West said including plasma Aβ42/40 would make the diagnosis more accurate among people in the earlier stages of amyloid deposition. In PARIS-IDEAS, some participants whose plaque load was at the bottom of the range of amyloid-PET positivity were detected as being amyloid-positive via plasma Aβ42/40, but not by p-tau217, he reported.
“If, in fact, the goal of a disease-modifying treatment strategy is to intervene as early in the disease process as possible among cognitively impaired individuals with AD, then having Aβ42/40 to aid in that earlier detection should be clinically impactful,” Braunstein wrote. He added that the combined biomarkers may become even more helpful as the field evolves toward preventing disease in cognitively normal people.
Test evaluation in the Swedish BioFinder cohort led to similar conclusions. Oskar Hansson of Lund University, Sweden, said that at different stages of AD, a different biomarker might best predict whether a person has brain amyloid. At the dementia stage, p-tau217 alone should be sufficient, whereas at the prodromal stage, p-tau217 might be paired with cognitive testing. At the preclinical stage, Aβ42/40 plus a measure of p-tau might prove most accurate at detecting amyloid plaques lurking in the brain. The question of which p-tau species might best detect early amyloid deposition at the preclinical stage is under intense investigation in several labs.
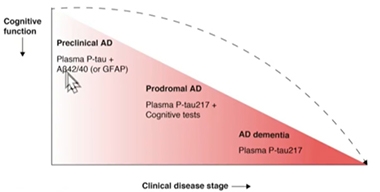
Biomarkers by Stage. As clinical disease worsens, fewer blood markers may be needed to detect brain amyloid pathology. [Courtesy of Oskar Hansson, Lund University]
In BioFinder, a comparison of different combinations of plasma biomarkers among cognitively normal participants showed that WashU’s mass spec blood Aβ42/40 ratio together with Eli Lilly’s blood p-tau217 assay detected amyloid with an AUC of 0.91. Either alone did so at AUC 0.84.
Hansson also presented a side-by-side comparison of 10 plasma p-tau assays measuring different phospho-epitopes and using different measurement platforms among people at the MCI stage. Wash U’s mass spec assay was the most accurate, with an AUC of 0.95, while Lilly and Janssen’s assays, which use meso-scale discovery (MSD) and Simo immunoassays, respectively, also performed well, posting AUCs approaching 0.9. Regardless of the type of assay, p-tau217 outperformed p-tau181 or p-tau231 in detecting amyloid at the MCI stage.
To move from learnings in cohorts toward enabling prognosis in individual patients, the Lund researchers are weaving these biomarker findings into algorithms that include ApoE status and cognitive testing (Janelidze et al., 2021; Palmqvist et al., 2022).
The superiority of the mass spec-based p-tau217 assay jibes with what was previously reported for plasma Aβ42/40, where mass spec assays led the field in amyloid detection (Oct 2021 news). Other assays are nipping at mass spec's heels. “We now have assays that perform remarkably well. This will revolutionize diagnostics in clinics,” Hansson said.
In an encouraging sign of converging evidence, Marc Suárez-Calvet of Hospital Del Mar Medical Research Institute and Barcelonaβeta, both in Barcelona, Spain, came to a similar conclusion. In San Diego, Suarez-Calvet presented a head-to-head comparison of nine plasma p-tau biomarker assays in a longitudinal cohort that included patients with neurodegenerative diseases who visited his hospital and underwent lumbar puncture. This real-world cohort is heterogenous. Participants have received different clinical diagnoses, including MCI, AD dementia, vascular dementia, dementia with Lewy bodies, progressive supranuclear palsy, and primary progressive aphasia. Instead of amyloid-PET, Suárez-Calvet relied upon the CSF ratio of Aβ42/p-tau as the determinant of brain amyloid positivity.
Suárez-Calver’s lineup consisted of Janssen’s p-tau217; Lilly’s p-tau181, p-tau217, and total tau; Quanterix’s p-tau181; the University of Gothenburg’s in-house p-tau181 and p-tau231 assays; as well as p-tau181 and p-tau217 assays from ADx Neurosciences, a Belgian assay developer acquired this summer by Fujirebio (Bloomberg news). Suárez-Calvet's team evaluated the performance of each assay using plasma and CSF from 197 participants, including 127 who were amyloid-positive based on their CSF Aβ42/p-tau ratio.
In short, the scientists found that several plasma assays were highly accurate at distinguishing between AD and non-AD CSF status in this memory clinic cohort. With an AUC of 0.96, Janssen’s p-tau217 came in first in this comparison, while ADx’s p-tau181 and Lilly’s p-tau217 assays were close behind at 0.94. Quanterix’s p-tau181 assay discriminated amyloid from non-amyloid CSF profiles with an AUC of 0.8, while Gothenburg’s p-tau231 assay clocked in at 0.88.
Suárez-Calvet concluded that, at least among people at this Barcelona hospital, several plasma p-tau assays work well on their own at pinpointing who has amyloid in their brain. He also believes more work is needed to see how these results apply to different populations, and that different marker combinations might do better at different stages of disease. For example, p-tau231 might prove most sensitive among people with early stage amyloid deposition who are cognitively normal. Recently published studies and presentations at AAIC suggest that plasma p-tau231 rises before p-tau217 in response to amyloid accumulation.
The field seems to have no shortage of plasma biomarker assays that closely track with amyloid status. But a test's accuracy is far from the only factor to consider when rolling it out around the globe. For one, tests need to be scalable and accessible, Hansson noted. While mass spec tests appear to be the most sensitive of the lot, they are costly and require specialized facilities and highly trained technicians to run. According to Braunstein, C2N is currently able to process several thousand per week in its CAP-CLIA facility and is partnering with other mass spec laboratories to boost capacity.
Immunoassays could be more practicable for use on a mass scale but even these have limitations, said Edward Wilson of Stanford University. He noted that some tests are plate-based, meaning that the test is run on proprietary plates preloaded with the reagents. These are vulnerable to variation between plates, he said, and are not amenable to adding in other biomarkers. He also noted that some tests are incapable of running at high throughput, and require expertise of experimenters or specialized equipment. Typically in medical care, blood tests that are widely used in routine clinical care have been fully automated as part of their clinical standardization and certification process.
Wilson tried out Fujirebio’s Lumipulse platform and plasma p-tau181 assay in the Stanford ADRC and Stanford Aging and Memory Study (SAMS), longitudinal studies that collect cognitive, genomic, and biomarkers data on their participants. All components of this assay are commercially available, high-throughput, fully automated, and run on a mid-size desktop analyzer. Wilson found that the assay differentiated amyloid-positive from -negative participants, with increasing separation between groups as their clinical disease stages worsened. The test posted an AUC of 0.95 for distinguishing amyloid-negative, cognitively unimpaired and amyloid-positive symptomatic people, though Wilson acknowledged that this is the lowest-hanging fruit in terms of discrimination. Future studies will compare the Lumipulse assay head-to-head with others in the field, Wilson said.
Much of the blood-biomarker data at AAIC focused on Aβ and p-tau. Is that all scientists need? Or would other analytes add value for prognosis? Consider GFAP. Astrocytes crank up expression of this cytoskeletal protein in response to neuronal damage, as well as in the vicinity of amyloid plaques. Teunissen used data from the Netherlands Twin registry, a population-based cohort that includes pairs of monozygotic twins, to investigate the predictive value of plasma biomarkers, including GFAP, for amyloid-positivity in people approaching their 60s.
At baseline, when participants were still cognitively normal and averaged 68 years of age, they were tested for amyloid by CSF or PET. They had already given blood samples 10 years prior. In San Diego, Teunissen reported that among those who were amyloid positive at baseline, blood levels of p-tau181 and GFAP already had been elevated a decade before and rose sharply thereafter. What’s more, twins who were concordant for amyloid at baseline also tended to be concordant for p-tau181 and GFAP. Curiously, even twins who were mismatched for amyloid were concordant for GFAP. To Teunissen, this concordance suggests that shared environmental or genetic factors influence GFAP levels, suggesting this astrocytic protein could be some sort of a predisposition marker.
Among people with brain amyloid, plasma p-tau181 and GFAP tracked with subsequent cognitive decline on memory tests, with GFAP having the strongest link. These twin cohort findings jibed with recently published findings from cognitively normal participants in the Amsterdam Dementia Cohort, where plasma GFAP and, to a lesser extent, plasma NfL, correlated with future conversion to MCI (Verberk et al., 2021).
At AAIC, Teunissen reported that in the DIAN cohort, plasma GFAP begins to rise in mutation carriers about 10 years prior to their expected symptom onset. Unlike p-tau181, which levels off after symptoms emerge, GFAP continued to rise for years as symptoms worsened. GFAP also correlated with subsequent brain atrophy among mutation carriers in the DIAN cohort, Teunissen reported. The findings cast GFAP as both a predictive marker that symptoms lie ahead and cognition will decline, and as a marker of progression. “This indicates that there is really added value of GFAP, in the early and late stages of the disease,” Teunissen said.
Teunissen agreed with other investigators that despite the rapidly growing, and largely convergent, data demonstrating the power of blood tests for the diagnosis and prognosis of AD, much work remains before they will wend their way into practice, especially primary care. For more on the road to get there, see the next part of this series (Part 3).—Jessica Shugart
References
News Citations
- Finally, a Blood Test for Alzheimer’s?
- Alzheimer's Blood Tests Have Arrived; Road to Broad Use Still Stretches On
- In Side-by-Side Test of 8 Blood Aβ Assays, Mass Spec Shines
Paper Citations
- Hu Y, Kirmess KM, Meyer MR, Rabinovici GD, Gatsonis C, Siegel BA, Whitmer RA, Apgar C, Hanna L, Kanekiyo M, Kaplow J, Koyama A, Verbel D, Holubasch MS, Knapik SS, Connor J, Contois JH, Jackson EN, Harpstrite SE, Bateman RJ, Holtzman DM, Verghese PB, Fogelman I, Braunstein JB, Yarasheski KE, West T. Assessment of a Plasma Amyloid Probability Score to Estimate Amyloid Positron Emission Tomography Findings Among Adults With Cognitive Impairment. JAMA Netw Open. 2022 Apr 1;5(4):e228392. PubMed.
- Janelidze S, Palmqvist S, Leuzy A, Stomrud E, Verberk IM, Zetterberg H, Ashton NJ, Pesini P, Sarasa L, Allué JA, Teunissen CE, Dage JL, Blennow K, Mattsson-Carlgren N, Hansson O. Detecting amyloid positivity in early Alzheimer's disease using combinations of plasma Aβ42/Aβ40 and p-tau. Alzheimers Dement. 2021 Jun 20; PubMed.
- Palmqvist S, Stomrud E, Cullen N, Janelidze S, Manuilova E, Jethwa A, Bittner T, Eichenlaub U, Suridjan I, Kollmorgen G, Riepe M, von Arnim CA, Tumani H, Hager K, Heidenreich F, Mattsson-Carlgren N, Zetterberg H, Blennow K, Hansson O. An accurate fully automated panel of plasma biomarkers for Alzheimer's disease. Alzheimers Dement. 2022 Aug 11; PubMed.
- Verberk IM, Laarhuis MB, van den Bosch KA, Ebenau JL, van Leeuwenstijn M, Prins ND, P, Teunissen CE, van der Flier WM. Serum markers glial fibrillary acidic protein and neurofilament light for prognosis and monitoring in cognitively normal older people: a prospective memory clinic-based cohort study. The Lancet Healthy Longevity, January 19, 2021 The Lancet Healthy Longevity
Other Citations
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.