After Long Wait, Aβ Oligomer Detangler Poised for Phase 2
Quick Links
While the trials, tribulations, and successes of Aβ immunotherapy were commanding Alzheimerologists' rapt attention, a different anti-amyloid approach has been quietly moving forward on the sidelines. PRI-002—a small molecule that disassembles Aβ oligomers—is about to start a sizeable Phase 2 trial. The announcement, along with a hint of a cognitive signal in a small Phase 1b trial, were presented at the Clinical Trials on Alzheimer’s Disease conference, held October 24 to 27 in Boston.
It is too early to draw conclusions about whether PRI-002, or the strategy of dismantling Aβ oligomers, will derail the early phases of AD pathogenesis. But the time is ripe to find out, according to Dieter Willbold of Heinrich-Heine-Universität Düsseldorf, Germany, who has ushered development of this drug for two decades. Unlike amyloid-targeted antibodies, which are costly, burdensome to administer, and come with risks such as ARIA, PRI-002 is a small molecule that can be taken as a capsule. Plus, it does not depend on the double-edged sword of the immune response to do its job, Willbold told Alzforum.
Identified in a screen more than 20 years ago, PRI-002, also known as Contraloid or RD2, is a 12-residue D-enantiomer peptide that interferes with assembly of Aβ42 oligomers (Wiesehan et al., 2003).
In 2017, Willbold and others co-founded Priavoid GmbH to develop the drug. Preclinical studies have shown that the compound readily crosses the blood-brain barrier and disassembles Aβ oligomers on the verge of prion-like mischief (e.g., Schemmert et al., 2018; May 2022 news; Kutzsche et al., 2023).
At CTAD, Oliver Peters of the Charité in Berlin presented an exploratory clinical outcome from a Phase 1b study. Top-line results had been reported at AAIC back in 2022. The trial was small, with a simple design: Seventeen people with MCI and two with mild AD were randomized to take daily 300 mg doses of PRI-002 or placebo for four weeks, then followed for another month. The drug appeared safe, at least over such a short period. Not only were there no serious adverse events, but more people in the placebo group had a mild adverse event than those on drug.
Relative to participants on placebo, those who took PRI-002 performed better on the CERAD word list, a measure of memory function. The effect reached significance one month after treatment stopped, Peters claimed. Memory scores improved in all nine participants on treatment; they improved in four of the 10 who received placebo.
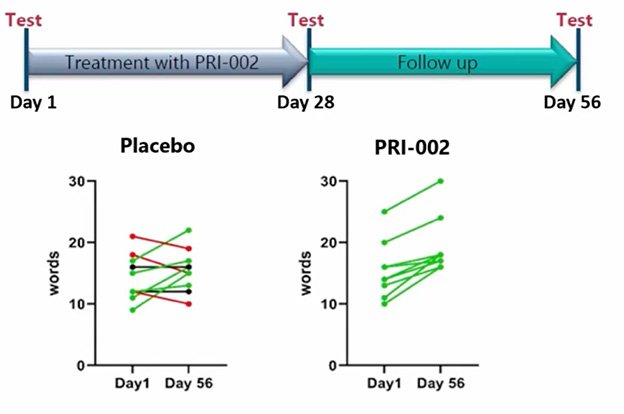
More Words on Drug? CERAD wordlist score improved for all participants who received PRI-002, compared to half who got placebo. [Courtesy of Oliver Peters, Charité Berlin.]
The trial also measured change on the CDR-SB. There were no differences between groups on this measure. Willbold said that the CDR-SB would not be expected to change after such a short treatment period.
A Phase 2 trial is slated to begin in early 2024. It will enroll at least 270 participants with MCI or mild AD at 40 trial sites in seven European countries. The trial will randomize volunteers to take daily doses of 300 mg or 600 mg PRI-002, or placebo, for one to two years. To generate information about treatment duration effects while also shortening the trial, Willbold said that earlier enrollees will be treated for the full two years, while later recruits will only go for a year. Change in CDR-SB will serve as the primary efficacy endpoint. Though this measure did not budge in Phase 1, it would be expected to do so in a longer trial, and is a standard outcome in future, pivotal studies, Willbold said. The trial is funded by SPRIN-D, the German Federal Agency of Disruptive Innovation.
After Peters' talk, Benjamin Wolozin of Boston University asked if similar cognitive benefits had been observed among healthy volunteers in earlier dosing studies, as this could address the question of whether the effects of the compound are due to its interaction with Aβ oligomers, or perhaps some other target. Priavoid conducted a single-ascending- and a multiple-ascending-dose Phase 1 trial prior to this one, but Peters said cognition was not tracked in healthy participants. Peters noted that the drug did lower CSF Aβ oligomers in the Phase 1b trial, suggesting that it hit its target. Echoing Wolozin, Robert Rissman of the University of California, San Diego, noted that PRI-002’s apparent CERAD benefit emerging after a short treatment period could imply that it exerts a more general rather than a disease-specific effect. This key question will be addressed in the longer, larger Phase 2 trial, Peters said.
Willbold told Alzforum that he would not expect to see a benefit in cognitively healthy people, because no relevant off-targets of PRI-002 had been identified in receptor screens.
Despite years of drug development effort, there are few FDA-approved D-peptide drugs. One is Octreotide, a synthetic form of the hormone somatostatin that has 2 D-amino acids in its structure. Additional experimental ones are being explored in indications including Covid, cancer, and Alzheimer's, for example, one against tau (Aillaud et al., 2022).—Jessica Shugart
References
News Citations
Paper Citations
- Wiesehan K, Buder K, Linke RP, Patt S, Stoldt M, Unger E, Schmitt B, Bucci E, Willbold D. Selection of D-amino-acid peptides that bind to Alzheimer's disease amyloid peptide abeta1-42 by mirror image phage display. Chembiochem. 2003 Aug 4;4(8):748-53. PubMed.
- Schemmert S, Schartmann E, Zafiu C, Kass B, Hartwig S, Lehr S, Bannach O, Langen KJ, Shah NJ, Kutzsche J, Willuweit A, Willbold D. Aβ Oligomer Elimination Restores Cognition in Transgenic Alzheimer's Mice with Full-blown Pathology. Mol Neurobiol. 2018 Jul 12; PubMed.
- Kutzsche J, Schemmert S, Bujnicki T, Zafiu C, Halbgebauer S, Kraemer-Schulien V, Pils M, Blömeke L, Post J, Kulawik A, Jürgens D, Rossberg WM, Hümpel M, Bannach O, Otto M, Araujo JA, Willuweit A, Willbold D. Oral treatment with the all-d-peptide RD2 enhances cognition in aged beagle dogs - A model of sporadic Alzheimer's disease. Heliyon. 2023 Aug;9(8):e18443. Epub 2023 Jul 29 PubMed.
- Aillaud I, Kaniyappan S, Chandupatla RR, Ramirez LM, Alkhashrom S, Eichler J, Horn AH, Zweckstetter M, Mandelkow E, Sticht H, Funke SA. A novel D-amino acid peptide with therapeutic potential (ISAD1) inhibits aggregation of neurotoxic disease-relevant mutant Tau and prevents Tau toxicity in vitro. Alzheimers Res Ther. 2022 Jan 21;14(1):15. PubMed.
Other Citations
External Citations
Further Reading
Papers
- Pauly T, Zhang T, Sternke-Hoffmann R, Nagel-Steger L, Willbold D. Differentiation of subnucleus-sized oligomers and nucleation-competent assemblies of the Aβ peptide. Biophys J. 2023 Jan 17;122(2):269-278. Epub 2022 Dec 17 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.