ApoE and Tau: Unholy Alliance Spawns Neurodegeneration
Quick Links
ApoE4 doesn’t need to interact with Aβ to wreak havoc in the brain. Tau tangles can bring out the apolipoprotein’s bad side too, according to findings presented at the 13th International Conference on Alzheimer’s and Parkinson’s Diseases, held March 29-April 2 in Vienna. David Holtzman of Washington University in St. Louis described a nexus of destruction in P301S-tau mice, reporting massive neurodegeneration in those that expressed the human ApoE4 allele. Without amyloid there, how would this work? The researchers found that ApoE4 jacked up the microglial response to tau pathology, which may have transformed astrocytes from neuronal supporters into neurotoxic entities. While the cause and effect relationships between these players are still fuzzy, it may soon be possible to define them in cell culture, as other researchers at the meeting reported they could generate bona fide astrocytes and microglia from human induced pluripotent stem cells (iPSCs).
Eloise Hudry of Massachusetts General Hospital in Boston commented that Holtzman’s work addressed a crucial missing link in the neurodegenerative disease field, i.e., the relationship between ApoE and tau. “This pioneering work stimulates people to ask more questions,” she told Alzforum. She added that the findings add fuel to growing excitement about new functions of ApoE, including its role as a transcription factor and in synaptic pruning (see Sep 2016 news; Jan 2017 news).
ApoE is known for influencing production and clearance of Aβ (see Jul 2011 webinar; Apr 2013 news; May 2014 news). However, several studies hint that the apolipoprotein may also affect neurodegenerative processes—to which tau pathology is closely linked—independently of Aβ. For example, AD patients carrying the ApoE4 allele generally have more tau and p-tau in the cerebrospinal fluid, even after correcting for their Aβ42 levels (see Apr 2013 news; Deming et al., 2017). ApoE4 also generally comes with greater brain atrophy in people with frontotemporal dementia, many of whom have tau but not Aβ pathology (see Agosta et al., 2009). Given the close relationship between tau pathology and neurodegeneration, Holtzman hypothesized that different ApoE isoforms may respond to or influence tau pathology differently.
To investigate this, graduate student Yang Shi crossed P301S mice, which express a tangle-forming FTD mutant of tau, to various ApoE knock-ins, as well as to ApoE knockout mice. To Shi and Holtzman’s great surprise, P301S mice expressing ApoE4 had profound neurodegeneration by nine months of age. Their brains were 25 percent smaller than those of the ApoE3 or ApoE2 mice. Animals without ApoE barely had neurodegeneration, suggesting that all ApoE alleles to some degree promote neuronal death in the presence of tauopathy.
Hypothesizing that ApoE4 may ramp up tau accumulation, Shi ran various biochemical and histopathological experiments to measure tau in the different mouse strains. However, she found only minor isoform-dependent differences between them, certainly not enough to explain the neurodegenerative effect, Holtzman said.
Next, Shi checked for a role of neuroinflammation in the degenerative cascade. She measured gene expression in microglia from nine-month-old P301S mice. Compared to microglia from P301S/ApoE knockout animals, microglia from P301S mice expressing any of the three human ApoE isoforms upregulated a suite of pro-inflammatory genes, and downregulated homeostatic ones. The skew toward inflammation was most dramatic in P301S/ApoE4 mice. Among the elevated proinflammatory genes were IL-1α, TNFα, and C1q—three microglial genes that a study led by Ben Barres at Stanford University recently implicated in turning normally neurotrophic astrocytes into killers (see Jan 2017 news). Holtzman therefore collaborated with Barres to measure gene expression of astrocytes in the various P301S mice. Ultimately, they found that the gene expression profile of astrocytes from P301S/ApoE4 mice fitted that of neurotoxic “A1” astrocytes previously identified by Barres.
Berislav Zlokovic at University of Southern California, Los Angeles, was excited by the convergence of tau, ApoE, and neuroinflammation in this work. That these factors conspire to cause neurodegeneration in the absence of Aβ is not surprising, he said, nor does it exclude a contribution of Aβ to the AD cascade. He noted that ApoE4 also compromises the integrity of the vascular system in the brain, which in turn can cause tau pathology (see May 2012 news). A compromised blood-brain barrier can activate microglia and astrocytes, placing vascular problems at the scene of the crime as well.
Holtzman proposed a model in which ApoE4 exacerbates the microglial response to tau aggregates, which in turn goads astrocytes into harming neurons. It is unclear how E4 inflames microglia more than do other ApoE alleles, Holtzman said. However, if ApoE4 triggers damaging neuroinflammatory responses to tau pathology, it could accelerate disease progression in AD as well as in primary tauopathies, a possibility Holtzman is investigating. Whether microglia in P301S mice are directly responsible for activating astrocytes is also unclear at the moment, but this is a question cell culture models may elucidate.
To that end, Julia TCW, a postdoc in Alison Goate’s lab at Mount Sinai School of Medicine in New York, presented her latest findings in astrocytes generated from human iPSCs. The researchers generated 42 astrocyte lines derived from 30 people, including men and women, as well as healthy controls and people with AD. The cells behaved similarly to primary astrocytes harvested directly from the human brain, gobbling up myelin in response to glutamate, TCW reported. They also appeared to exist in a quiescent, resting state unless given a stimulus. This quiescence was of utmost importance, TCW stressed, as hyper-reactivity is a common problem with primary astrocytes isolated from the human brain.
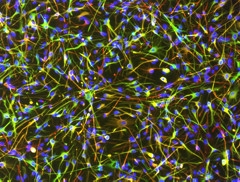
Bona Fide Astrocytes.
Star-shaped astrocytes differentiated from human iPSCs thrived in a quiescent state after differentiation. (Green: S100B; Red: GFAP; Blue: DAPI.) [Image courtesy of Julia TCW.]
Interestingly, astrocytes generated from people carrying two copies of ApoE4 expressed higher resting-state levels of the inflammatory cytokines IL-6 and IL-8 than did cells from people with an E3/E3 genotype. Treatment of astrocytes with Aβ42 or tau oligomers boosted cytokine secretion regardless of ApoE genotype, however, E3/E3 astrocytes had a larger increase in cytokine secretion, perhaps owing to their lower baseline levels, TCW said.
While Holtzman proposed that ApoE may primarily modulate astrocyte activation indirectly via microglia, TCW’s work suggests that ApoE isoforms also directly affect astrocytes in both resting and activated states. TCW hypothesized that cross talk between microglia and astrocytes occurs within the complex cellular milieu of the brain, although cause and effect relationships are difficult to unravel. She also noted that ApoE’s lipidation state—which differs between mice and humans—influences its activity. This makes human iPSC-derived models more relevant to understanding how ApoE affects neuroinflammation, she added.

Man-made Microglia.
An iPSC-derived microglial cell fans out its processes following transplantation into mice. (Red: human cytoplasm marker SC121; green: resting microglial marker P2RY12; blue: Iba1.) [Image courtesy of Mathew Blurton-Jones.]
Completing the iPSC menagerie, Mathew Blurton-Jones of the University of California, Irvine, described the differentiation of microglia from the pluripotent cells. Graduate student Edsel Abud used a two-step differentiation procedure to mimic the conditions that the hemotopoietic cells first experience in the yolk sac, and then in the brain, as they develop into microglia. RNA sequencing revealed that the resulting cells expressed a similar homeostatic suite of genes as do human microglia at rest.
When treated with Aβ fibrils or tau oligomers derived from postmortem brain samples of AD patients, the microglia upregulated ApoE, as well as several genes associated with AD risk in genome-wide association studies. The cells also ramped up expression of neuroinflammatory genes, including the same troublesome triad—IL-1α, C1q, TNFα—that fire up A1 astrocytes. The researchers injected the microglia into neuronal organoids, and also into the brains of immunodeficient mice. Strikingly, the microglia migrated and took positions at regular intervals throughout the organoids and mouse brains, much as endogenous microglia tile throughout the brain.
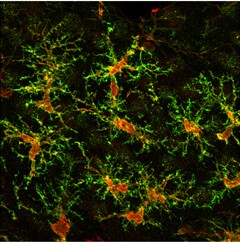
Taking Position.
Microglia differentiated from human iPSCs tile across a mouse brain after transplantation. (Red: human cytoplasm marker SC121; green: microglial marker TMEM119.) [Image courtesy of Mathew Blurton-Jones.]
Blurton-Jones is collaborating with researchers in Goate’s lab to put iPSC-derived astrocytes into the organoid mix as well. He commented to Alzforum that it would be interesting not only to co-culture neurons, microglia, and astrocytes derived from the same person’s iPSCs, but also to mix and match cells with different ApoE genotypes. This could show which cells deliver the ApoE4 that activates microglia, and test whether the microglia truly turn astrocytes into killers. TCW and colleagues are now using CRISPR gene editing to generate isogenic lines in which only the ApoE genotype is changed. That way she can focus on the role of ApoE without having to deal with the influence of differing genetic background.—Jessica Shugart
References
News Citations
- ApoE Variants Modulate Astrocyte Appetite for Synapses
- ApoE Risk Explained? Isoform-Dependent Boost in APP Expression Uncovered
- ApoE Does Not Bind Aβ, Competes for Clearance
- Has ApoE’s Time Come as a Therapeutic Target?
- By Digging Tau, GWAS Unearths TREM (Again), Plus New Leads
- Microglia Give Astrocytes License to Kill
- ApoE4 Makes Blood Vessels Leak, Could Kick Off Brain Damage
Webinar Citations
Research Models Citations
Paper Citations
- Deming Y, Li Z, Kapoor M, Harari O, Del-Aguila JL, Black K, Carrell D, Cai Y, Fernandez MV, Budde J, Ma S, Saef B, Howells B, Huang KL, Bertelsen S, Fagan AM, Holtzman DM, Morris JC, Kim S, Saykin AJ, De Jager PL, Albert M, Moghekar A, O'Brien R, Riemenschneider M, Petersen RC, Blennow K, Zetterberg H, Minthon L, Van Deerlin VM, Lee VM, Shaw LM, Trojanowski JQ, Schellenberg G, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Peskind ER, Li G, Di Narzo AF, Alzheimer’s Disease Neuroimaging Initiative (ADNI), Alzheimer Disease Genetic Consortium (ADGC), Kauwe JS, Goate AM, Cruchaga C. Genome-wide association study identifies four novel loci associated with Alzheimer's endophenotypes and disease modifiers. Acta Neuropathol. 2017 May;133(5):839-856. Epub 2017 Feb 28 PubMed.
- Agosta F, Vossel KA, Miller BL, Migliaccio R, Bonasera SJ, Filippi M, Boxer AL, Karydas A, Possin KL, Gorno-Tempini ML. Apolipoprotein E epsilon4 is associated with disease-specific effects on brain atrophy in Alzheimer's disease and frontotemporal dementia. Proc Natl Acad Sci U S A. 2009 Feb 10;106(6):2018-22. PubMed.
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.