BACE Inhibition and the Synapse—Insights from Seeon
Quick Links
Potential side effects remain a major concern for BACE inhibitor programs. Knocking out the gene for BACE1 in mice causes a variety of troubling phenotypes, not least being brain seizures (see Part 2 of this series). Among the more than 40 potential substrates for BACE1 in the proteome, several are likely relevant to seizure activity. At the 2nd Kloster Seeon meeting on BACE proteases, held September 25-27 near Munich, three stood out. They are seizure protein 6 (SEZ6), neuregulin 3, and MDGA1, a neuronal cell adhesion molecule linked to autism and schizophrenia.
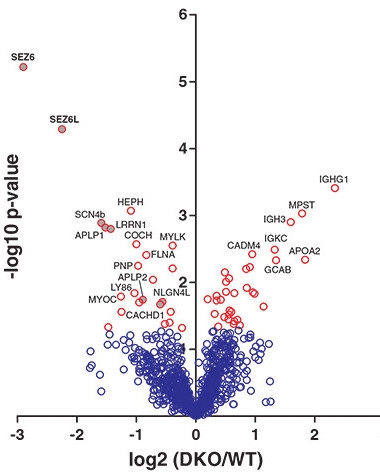
Proteomic Analysis Lifts Lid on BACE Substrates.
This “volcano” plot shows that levels of SEZ6 and SEZ6L ectodomains (x axis) are significantly (y axis) lower in CSF of BACE1/2 double knockout mice than in CSF from wild-type animals. [Courtesy of Pigoni et al., 2016.]
Researchers led by meeting co-organizer Stefan Lichtenthaler of the German Center for Neurodegenerative Diseases in Munich had previously identified the transmembrane protein SEZ6 as a potential BACE1 substrate (see Kuhn et al., 2012; Dec 2013 conference news). In Seeon, Lichtenthaler reported that shedding of SEZ6’s ectodomain plummeted eightfold both in BACE1 and in BACE1/2 knockout mice (see volcano plot at right) but not in BACE2 knockouts, fingering BACE1 as a major SEZ6 sheddase (Pigoni et al., 2016).
This held up in primates, as well. Analyzing rhesus monkey CSF samples after giving the animals a single dose of the BACE inhibitor MBI-4, the researchers found a reduction in SEZ6 ectodomain levels compared to samples taken after the same animal was given vehicle alone.
Could blocking the BACE1 cleavage of SEZ6 in adult animals be deleterious? To investigate, researchers in Jenny Gunnersen’s lab at the University of Melbourne, Australia, made SEZ6 conditional knockout mice. They expressed cre recombinase driven by a tamoxifen-inducible CamKII promoter in the animals, together with a floxed SEZ6 gene. In Seeon, Gunnersen showed that tamoxifen reduced SEZ6 expression in the CA1 region of the hippocampus and in the cortices of adult mice. This changed the animals’ behavior. They froze more easily in a contextual fear conditioning paradigm, suggesting they remembered better, even up to seven days after training.
Knocking out all three members of the SEZ6 family (i.e., SEZ6-like and SEZ6-like 2) seems to subtly affect memory as well (Miyazaki et al., 2006). In collaboration with Hiroshi Takeshima at Kyoto University, Japan, Gunnersen found that the triple KOs swam more slowly than controls, but learned to find the hidden platform in a water maze just as quickly. “This might be a sign that they initially learn slightly faster,” suggested Gunnersen. But in a reversal test, where the platform was moved to a new location, the mice took much longer to catch on. Gunnersen thinks cognition in these animals is rigid for some reason. This was also apparent in contextual fear conditioning. Here, too, the triple KOs seemed to learn better at first, freezing more readily when placed in a cage they associated with a foot shock, but while controls lost that memory over a few days, the triple KOs retained it.
It’s unclear what might underlie these effects. Gunnersen is looking for alterations in a number of brain regions, including the hippocampus and prefrontal cortex. As per electrophysiology, the paired-pulse ratio, which reflects synaptic vesicle release, was normal in the hippocampi of adult SEZ6 conditional knockouts, but excitatory currents were weaker, pointing to postsynaptic defects. In keeping with this, Gunnersen found that their synaptic spine heads were smaller. She said that soluble forms of SEZ6 may act like thrombospondin, which promotes synaptogenesis, and showed that secreted SEZ6 promotes excitatory synapse development similarly to thrombospondin in a model neuron culture system.
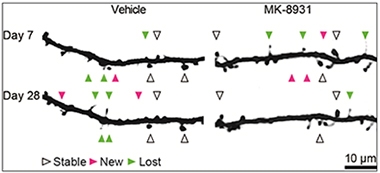
Spine Dynamics.
When given MK-8931 for three weeks, mice made fewer new dendritic spines and their spine density dropped. [Courtesy of Jochen Herms.]
Further evidence that healthy synapses might require SEZ6 came from Jochen Herms and colleagues at Ludwig Maximilians University, Munich. Previously, Herms had reported that BACE inhibitors impeded the formation of dendritic spines. Using two-photon microscopy to peer through a cranial window into the mouse brain, the researchers had recorded spine dynamics after administering high doses of either SCH1682496 or LY2811376, discontinued BACE inhibitors from Merck and Eli Lilly, respectively. In both cases the formation of new spines plummeted by half, and the overall numbers of spines fell 10 percent over a short period (see Nov 2014 news). In Seeon, Herms said that they have since seen the same effect with other BACE inhibitors, including MK-8931, which is being tested in Phase 2/3 clinical trials. After 21 daily doses of this drug, they observed a significant decrease in spine density due to impairment of new spine formation. Both spine formation and density bounced back once the drug was withdrawn. Interestingly, Herms does not see this effect in SEZ6 knockouts, suggesting this substrate may mediate the impact of BACE on spine turnover.
Herms also reported that knocking out SEZ6 caused postsynaptic deficits, as evidenced by reduced long-term potentiation. NB-360, another BACE inhibitor, had a similar effect, but not in SEZ6 knockouts, said Herms, again suggesting that BACE processing of SEZ6 plays a role in synaptic plasticity.
Interestingly, Lichtenthaler believes the BACE1 substrate MDGA1 may act at the postsynapse, as well. Researchers in his lab found more MDGA1 in whole brain extracts of BACE1 knockouts, suggesting BACE1 normally processes this membrane protein. In fact, Lichtenthaler reported that the BACE1 cleavage sites on MDGA1 and SEZ6 are very similar, and that neurons shed less soluble MDGA1 when BACE1 is knocked down or blocked pharmacologically.
What might be the consequences of blocking BACE1 cleavage of MDGA1? Others have shown that overexpressing MDGA1 prevents postsynaptic neuroligin-2 from interacting with presynaptic neurexin, thereby suppressing the formation of inhibitory synapses (see Pettem et al., 2013; Lee et al., 2013). If blocking BACE1 caused MDGA1 to accumulate, the effect might be the same. In keeping with this, Lichtenthaler’s group found that the BACE inhibitor C3 reduced the amount of the vesicular GABA transporter, VGAT, in mouse brain. GABA is the major inhibitory neurotransmitter in the central nervous system, and VGAT localizes to GABAergic synapses.
Evidence that BACE plays a role at the other side of the synaptic divide—the presynapse—came from Carmen Birchmeier, at the Max-Delbrück Center for Molecular Medicine, Berlin. Thomas Muller in her lab found that BACE1 not only cleaves neuregulin 3 (Nrg3), as had been reported by others (see Hu et al., 2008), but also stabilizes it on the neuron surface. This came as a surprise to Birchmeier and to others at the meeting, since many scientists have come to think of BACE cleavage as synonymous with ectodomain shedding. In fact, Birchmeier had previously found that BACE1 processed an isoform of neuregulin1 (Nrg1) in this manner and that this shedding was necessary for the proper formation of muscle spindles (Cheret et al., 2013). She reported that the protease, together with other sheddases, releases the Nrg1 extracellular domain, which then goes on to bind cell surface receptors (see July 2013 news). In Seeon, Birchmeier reported that BACE1 seems to cleave Nrg3 only at one site, which means the extracellular domain remains attached to the cell.
Birchmeier showed that Nrg3 occurs at synapses that express ErbB4. Specifically, she said Nrg3 in excitatory presynapses binds ErbB4 in postsynaptic membranes on inhibitory neurons. In fact, while Nrg3 knockout mice seem normal at first blush, they are a little hyperactive, but less impulsive, and mimic some symptoms of schizophrenia (see Loos et al., 2014; Hayes et al., 2016).
Could blocking or knocking out BACE1 have a similar effect to knocking out Nrg3? Cell culture experiments suggest yes. Birchmeier reported that BACE1 knockouts and cells expressing Nrg3 sans the extracellular domain had similar characteristics—namely, both had less ErbB4 in the postsynapse, and less synaptophysin in the presynapse. All told, Birchmeier thinks that by stabilizing Nrg3 at the cell surface, BACE1 helps strengthen excitatory synapses on inhibitory neurons.
Researchers were intrigued by these findings. On Lichtenthaler’s work, they noted how impressive the monkey CSF changes were after just one dose of BACE inhibitor. To the question of whether he would similarly analyze human CSF, Lichtenthaler said he would love to if he could get access to suitable samples. Others thought CSF “shedome” analysis might be valuable for companion diagnostics, a technique used to monitor drug efficacy in individual patients, asking if it could be used to test whether essential substrates of BACE1 were being spared by the drug at hand. Lichtenthaler considers it possible that cleavage of some substrates may be more strongly inhibited than others. “It might be interesting to see how far [CSF] Aβ falls while monitoring other substrates simultaneously. If they are not strongly reduced as well, we could then boost the dose of the inhibitor.”
Others were surprised that BACE seemed to have such a strong effect on the postsynapse. Robert Vassar, Northwestern University, Chicago, who co-organized the meeting with Lichtenthaler, said he had never even seen BACE there. Lichtenthaler agreed this was puzzling. “While many substrates are found on the postsynapse and BACE is seen on the presynapse I don’t think we fully appreciate where BACE1 cleavage occurs,” he said. “Not all BACE cleavage necessarily occurs in endosomes,” he added, suggesting the trans-Golgi might be a site for cleavage of postsynaptic membrane proteins. Vassar agreed, noting that BACE cleaves APP harboring the Swedish mutation on its way to the cell surface—it does not have to recycle through endosomes first.
Researchers further wondered if the soluble and membrane-bound forms of SEZ6 and MDGA1 have different functions and if the soluble forms can affect the presynapse. Gunnersen said the two forms of SEZ6 may have opposite functions, since overexpression of the membrane form inhibits dendritic branching, while the soluble form enhances it. She posited that the shed form interferes with protein-protein interactions of the membrane form and that this may help balance signaling events. She did not think that the soluble SEZ6 acts as a type of chemoattractant for the presynapse in development, though.
As for the presynapse, researchers wondered why Nrg3 levels fall if BACE does not cleave the protein. Birchmeier suggested that perhaps unprocessed Nrg3 never reaches the cell surface. “We are not sure yet,” she said, but noted that the loss of Nrg3 in the BACE1 knockouts is very obvious. Christian Haass, Ludwig-Maximilians University, Munich, wondered if Nrg3 and BACE1 translocated to the cell surface together as a dimer. Birchmeier thought that idea worth exploring.—Tom Fagan
References
News Citations
- What Exactly Does BACE Do in Adults?
- BACE—Substrates, Functions, Developmental Phenotypes
- At High Doses, BACE1 Inhibitors Hinder Synaptic Plasticity in Mice
- Paper Alert: BACE1 Required for Muscle Spindle, Motor Control
Therapeutics Citations
Paper Citations
- Kuhn PH, Koroniak K, Hogl S, Colombo A, Zeitschel U, Willem M, Volbracht C, Schepers U, Imhof A, Hoffmeister A, Haass C, Rossner S, Bräse S, Lichtenthaler SF. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 2012 Jul 18;31(14):3157-68. PubMed.
- Pigoni M, Wanngren J, Kuhn PH, Munro KM, Gunnersen JM, Takeshima H, Feederle R, Voytyuk I, De Strooper B, Levasseur MD, Hrupka BJ, Müller SA, Lichtenthaler SF. Seizure protein 6 and its homolog seizure 6-like protein are physiological substrates of BACE1 in neurons. Mol Neurodegener. 2016 Oct 5;11(1):67. PubMed.
- Miyazaki T, Hashimoto K, Uda A, Sakagami H, Nakamura Y, Saito SY, Nishi M, Kume H, Tohgo A, Kaneko I, Kondo H, Fukunaga K, Kano M, Watanabe M, Takeshima H. Disturbance of cerebellar synaptic maturation in mutant mice lacking BSRPs, a novel brain-specific receptor-like protein family. FEBS Lett. 2006 Jul 24;580(17):4057-64. Epub 2006 Jun 27 PubMed.
- Pettem KL, Yokomaku D, Takahashi H, Ge Y, Craig AM. Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development. J Cell Biol. 2013 Feb 4;200(3):321-36. Epub 2013 Jan 28 PubMed.
- Lee K, Kim Y, Lee SJ, Qiang Y, Lee D, Lee HW, Kim H, Je HS, Südhof TC, Ko J. MDGAs interact selectively with neuroligin-2 but not other neuroligins to regulate inhibitory synapse development. Proc Natl Acad Sci U S A. 2013 Jan 2;110(1):336-41. Epub 2012 Dec 17 PubMed.
- Cheret C, Willem M, Fricker FR, Wende H, Wulf-Goldenberg A, Tahirovic S, Nave KA, Saftig P, Haass C, Garratt AN, Bennett DL, Birchmeier C. Bace1 and Neuregulin-1 cooperate to control formation and maintenance of muscle spindles. EMBO J. 2013 Jun 21; PubMed.
- Loos M, Mueller T, Gouwenberg Y, Wijnands R, van der Loo RJ, Neuro-BSIK Mouse Phenomics Consortium, Birchmeier C, Smit AB, Spijker S. Neuregulin-3 in the mouse medial prefrontal cortex regulates impulsive action. Biol Psychiatry. 2014 Oct 15;76(8):648-55. Epub 2014 Feb 24 PubMed.
- Hayes LN, Shevelkin A, Zeledon M, Steel G, Chen PL, Obie C, Pulver A, Avramopoulos D, Valle D, Sawa A, Pletnikov MV. Neuregulin 3 Knockout Mice Exhibit Behaviors Consistent with Psychotic Disorders. Mol Neuropsychiatry. 2016 Jul;2(2):79-87. Epub 2016 May 20 PubMed.
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.