Sleep and Brain Cleansing—Fresh Insights into Regulation and Disruption
Quick Links
Throughout the arc of human history, sleep has been a mysterious process that has captured the imaginations of both artists and scientists. Researchers are slowly deciphering how sleep restores us, and how its woeful absence makes us ill. Scientists now think a good night’s rest benefits the brain by clearing away cellular waste and laying down memories. Recent studies on how these processes work may have implications for neurodegenerative disease, particularly Alzheimer’s. “There is an increasing awareness that we need to know more about how sleep is implicated in disease etiologies,” said Maiken Nedergaard at the University of Rochester Medical Center, New York, who led one of the studies.
This story explains how sleep and osmotic pressure are intimately connected, how poor fluid flow in the brain correlates with disease, and how brain injury may disturb sleep. (For new insights into the role of sleep in memory, see Part 2 of this series.)
Sleeping by Osmosis
Both the volume and flow of extracellular fluid are known to surge when the brain catches some zzz's. This extra flow facilitates the clearance of solutes including Aβ, according to one theory.
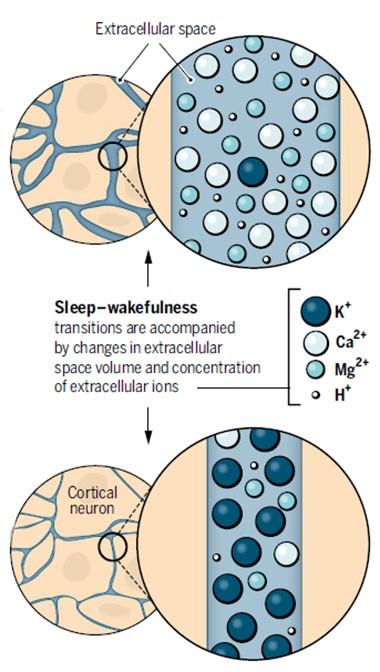
Night and Day.
During sleep (top), calcium and magnesium flood into the extracellular fluid and its volume swells, while in the waking brain, potassium predominates and volume shrinks (bottom). [Courtesy of Science/AAAS.]
Nedergaard wanted to know why there is more fluid in sleeping brains. Because osmotic forces control fluid movements, Nedergaard speculated that the answer might have something to do with the extracellular concentrations of ions and solutes. As reported in the April 29 Science, joint first authors Fengfei Ding and John O’Donnell and their colleagues tested this idea by adding a “wakeup” mix of neuromodulators to mouse cortical brain slices. The mix included norepinephrine, acetylcholine, and dopamine, which are produced by brainstem and basal brain regions and are known to stimulate waking patterns of electrical activity in the brain. The treatment induced a rapid and persistent rise in extracellular potassium in the slices. Neurons release this ion when they fire, but surprisingly, potassium spiked even when brain activity in the slices was silenced with tetrodotoxin. This demonstrated that the ion change occurred independently of electrical activity. It is not yet clear what caused the potassium efflux, Nedergaard said.
Intrigued, the authors measured ion concentrations in waking and sleeping mice via microdialysis. As in brain slices, potassium levels shot up within a few seconds of waking; they remained high until the animals nodded off, then plunged. These changes occurred even when electrical activity was dampened with an AMPA receptor antagonist. Meanwhile, calcium and magnesium ions waxed and waned in opposition to the potassium, rising during sleep and falling as animals awoke. Their transitions were a bit slower, taking about a minute apiece. The ion changes all occurred independently of each other, the scientists reported.
To tease out the effect of different ion concentrations, the authors infused ion cocktails into the brains of mice through a cranial window. When they added a “waking” mix of ions to one cortical region of a sleeping mouse, the neurons in that small region shifted into waking patterns of electrical activity, although the mouse remained asleep. Likewise, a “sleeping” mix added to awake animals induced slow-wave electrical activity characteristic of sleep in that teeny portion of cortex, even while the animals remained active. At the same time, the volume of local extracellular fluid shrank or expanded in keeping with the ion mix (see image above). This was determined by the classic method of infusing the cation tetramethylammonium and recording how quickly it diffused from the delivery site.
Then the authors went one step further. They changed the ion composition throughout the brain by infusing ion cocktails into the cerebrospinal fluid. With that, they found they could transition global brain activity, waking up sleeping mice and putting awake mice to sleep.
The findings suggest, at a simple level, that ion concentration itself controls sleep/wake in the brain, Nedergaard told Alzforum. The idea is not new; studies from the 1930s first proposed that calcium and magnesium promoted sleep, while potassium promoted wakefulness, noted Hans-Peter Landolt and Sebastian Holst at the University of Zurich, in an accompanying editorial. However, this idea fell into obscurity when subsequent research assumed that changes in electrical activity caused the ups and downs in ion concentrations during sleep and wakefulness. The new data imply the opposite, Nedergaard noted. As an animal falls asleep, extracellular potassium plummets first, hyperpolarizing and silencing neurons at the same time fluid volume swells to facilitate clearance of solutes. “When the brain needs to clean itself, it shuts itself off by lowering potassium,” Nedergaard suggested.
Brendan Lucey at Washington University in St. Louis found the data surprising. “I wouldn’t have thought that regulating ion concentrations in mouse CSF would result in state changes in the brain,” he told Alzforum. He noted that the study used careful controls and built its case well, moving from brain slices to live animals.
What Can Go Wrong
Nedergaard thinks the research may also provide clues to what goes awry in disease states. A number of studies have linked disrupted sleep to Alzheimer’s risk (see, e.g., Aug 2012 conference news; Oct 2013 news). Recent work indicates that Aβ exits the brain primarily during sleep, suggesting one possible reason for the association (see Sep 2009 news; Apr 2013 conference news; Jun 2014 news). Nedergaard and colleagues had previously proposed a mechanism for this clearance. They described a “glymphatic” flow that washes through the brain interstitial space, dragging Aβ and other solutes along with it. Specialized channels on astrocytes that pump water power the flow, which is much greater during sleep (see Aug 2012 news; May 2014 conference news). The new data suggest the increase in osmotic pressure that occurs during sleep may help power that current. “The data help establish a mechanism for Nedergaard’s prior observations that Aβ clearance occurs during sleep,” Lucey said.
Ion imbalance might underlie other symptoms as well. For example, Ding and colleagues found that in animals recovering from anesthesia, the ions lagged in returning to normal waking levels, with potassium taking a couple of minutes and calcium and magnesium requiring up to half an hour to stabilize. Mice in this state appeared disoriented, running into the walls of their cage. This slow transition may explain the grogginess common upon waking from anesthesia, Nedergaard speculated. Perhaps a similar ion perturbation causes some of the confusion seen in people with Alzheimer’s, particularly in the evenings, Nedergaard said. In ongoing work, she is measuring ion concentrations in the brains of aged AD model mice to test this idea. She is also interested in whether extracellular potassium levels continue to climb if animals are kept awake past their normal hour for shut-eye. High potassium is known to cause seizures, and might provide an explanation for why these occur after prolonged sleep deprivation, Nedergaard noted.
The findings might also point toward therapeutic targets, Landolt and Holst suggested. “Pumps and transporters that control ion flow across cell membranes may be promising new targets for treating sleep-wake disorders,” they wrote. For her part, Nedergaard noted that astrocytes express specific subtypes of sodium/potassium pumps, suggesting these could be selectively targeted.
Beyond Glymph—Sleep and Other Clearance Pathways
Glymphatic flow is but one of several complementary routes for clearing solutes from the brain. Researchers last year reported the discovery of lymphatic vessels in the dura mater that absorb fluid from interstitial fluid (ISF) and CSF and transport it to cervical lymph nodes (see Louveau et al., 2015; Aspelund et al., 2015). In addition, fluids can drain along the basement membranes of brain blood vessels, through the perivascular spaces. Enlargements in these Virchow-Robin spaces, as they are called, correlate with cardiovascular disease, stroke, cognitive problems, and dementia.
Researchers led by Joel Ramirez at the University of Toronto have associated low sleep quality with anatomical changes in these fluid-filled gaps. Along with Courtney Berezuk, Ramirez examined data from 26 adults with an average age of 60 who were evaluated for cardiovascular disease (see Berezuk et al., 2015). Participants with the largest Virchow-Robin spaces slept worst. They took longer to fall asleep, woke up more during the night, and spent less time in the third and deepest stage of non-REM sleep.
Why these spaces were enlarged remains unclear. The authors speculated that lack of sleep rendered drainage from perivascular spaces inefficient, which in turn could cause these channels to clog up with waste products such as Aβ and expand (see Weller et al., 2009). On the other hand, underlying cardiovascular disease by itself might be responsible for these spaces ballooning, regardless of sleep problems. One theory holds that as arteries harden, they beat more against surrounding glia and carve out a larger space, said Costantino Iadecola at Weill Cornell Medical Center, New York. The consequences of enlarged Virchow-Robin spaces are unknown. Berislav Zlokovic at the University of Southern California, Los Angeles, pointed out that it might influence the rate of solute clearance from the ISF to the CSF. However, Iadecola noted that remains to be proven (see full comment below).
Other studies support the idea that normal clearance mechanisms falter in AD. Researchers led by Tsutomu Nakada at the University of Niigata, Japan, reported that the flow of water from ISF into CSF slows down in older people, and lags even more in those with Alzheimer’s disease (see Suzuki et al., 2015). They compared fluid flow in 10 young volunteers, 10 healthy aged volunteers, and 10 people diagnosed with AD dementia, using PET imaging of water labeled with an oxygen isotope. The flow in AD patients was about half that in the younger people, with healthy elderly falling about halfway between the two groups.
John Cirrito at WashU noted that these findings fit with previous evidence from his WashU colleague Randy Bateman, who found that rather than spiking during sleep as they do in healthy adults, CSF Aβ levels remain steady throughout the circadian cycle in AD patients (see Dec 2010 news; Aug 2012 conference news; Huang et al., 2012). “Nakada’s study suggests the reason for flat Aβ levels is because the flow from ISF to CSF is impaired,” Cirrito said.
Beyond AD
Perturbed sleep shows up in other brain disorders as well. In the April 27 Neurology, researchers led by Lukas Imbach and Christian Baumann at University Hospital Zurich, reported that people with traumatic brain injury sleep more than controls even 18 months after their injury. The findings extended data from a previous study, where the authors reported increased sleepiness in a cohort of 42 young adults six months after TBI (see Imbach et al., 2015). At 18 months, the authors were able to obtain additional polysomnography data from 31 of those participants. Compared with 42 age-matched controls, the TBI patients slept about one hour more per night, but nonetheless remained sleepier during the day, as measured by how quickly they fell asleep for naps. Overall, two-thirds of the TBI patients were diagnosed with excessive daytime sleepiness, as compared to one-fifth of controls. Other sleep parameters appeared normal. The findings match the data at six months, indicating the changes in sleep patterns persisted.
Unlike at six months after their injury, at 18 months no correlation emerged between sleep problems and the severity of the injury. It may be that the need for extra sleep soon after injury serves a purpose in healing the brain, but over time sleep disturbances transform into a chronic, maladaptive condition, the authors speculated. Intriguingly, a recent study from the same group found that when rats were induced to sleep more in the first two weeks after a traumatic brain injury, they maintained their ability to remember new objects, whereas controls did not. These power sleepers expressed less APP in the hippocampus and cortex than injured controls did, suggesting the extra sleep might mitigate axonal injury (see Morawska et al., 2016). However, it remains to be determined whether the extra sleep TBI patients get more than a year after injury helps or harms their brains.
Notably, these patients did not realize they had a sleep problem, as their self-reports and clinical interviews regarding sleep habits resembled those of controls. This suggests sleep disorders may go unrecognized in TBI patients, and therefore untreated. Such patients should be evaluated by objective sleep laboratory measures, the authors suggest.
“Imbach et al. make a compelling case that post-traumatic sleep-wake disorders may represent a silent epidemic,” wrote Brian Edlow at Massachusetts General Hospital, Boston, and Gert-Jan Lammers at Leiden University Medical Centre, the Netherlands, in an accompanying editorial. They noted that the study represents the most comprehensive longitudinal analysis of post-traumatic sleep-wake disturbances to date.
Would sleep therapy help TBI patients, or people with AD for that matter? No one knows. “We need very good controlled, prospective studies on how sleep therapy (not sleep medications) can reduce the progression of different diseases, including Alzheimer’s,” Nedergaard said.—Madolyn Bowman Rogers
References
News Citations
- In mTORC and Theta Rhythms, New Clues to How Sleep Locks Down Memories
- Night Owl? Early Bird? Good Night’s Sleep May Protect the Brain
- From ApoE to Zzz’s—Does Sleep Quality Affect Dementia Risk?
- Sleep Deprivation Taxes Neurons, Racks Up Brain Aβ?
- Sleep Patterns, Circadian Clock Linked to Aβ Oxidative Stress
- While You Were Sleeping—Synapses Forged, Amyloid Purged
- Brain Drain—“Glymphatic” Pathway Clears Aβ, Requires Water Channel
- Glymphatic Flow, Sleep, microRNA Are Frontiers in Alzheimer’s Research
- Paper Alert: In Vivo Human Data Shows Reduced Aβ Clearance in AD
- Plaque May Quash Seesawing CSF Aβ Levels
Paper Citations
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 Jul 16;523(7560):337-41. Epub 2015 Jun 1 PubMed.
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015 Jun 29;212(7):991-9. Epub 2015 Jun 15 PubMed.
- Berezuk C, Ramirez J, Gao F, Scott CJ, Huroy M, Swartz RH, Murray BJ, Black SE, Boulos MI. Virchow-Robin Spaces: Correlations with Polysomnography-Derived Sleep Parameters. Sleep. 2015 Jun 1;38(6):853-8. PubMed.
- Weller RO, Boche D, Nicoll JA. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer's disease and their potential impact on therapy. Acta Neuropathol. 2009 Jul;118(1):87-102. PubMed.
- Suzuki Y, Nakamura Y, Yamada K, Igarashi H, Kasuga K, Yokoyama Y, Ikeuchi T, Nishizawa M, Kwee IL, Nakada T. Reduced CSF Water Influx in Alzheimer's Disease Supporting the β-Amyloid Clearance Hypothesis. PLoS One. 2015;10(5):e0123708. Epub 2015 May 6 PubMed.
- Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju YE, Kasten T, Morris JC, Mintun M, Duntley S, Bateman RJ. Effects of age and amyloid deposition on aβ dynamics in the human central nervous system. Arch Neurol. 2012 Jan;69(1):51-8. PubMed.
- Imbach LL, Valko PO, Li T, Maric A, Symeonidou ER, Stover JF, Bassetti CL, Mica L, Werth E, Baumann CR. Increased sleep need and daytime sleepiness 6 months after traumatic brain injury: a prospective controlled clinical trial. Brain. 2015 Mar;138(Pt 3):726-35. Epub 2015 Jan 15 PubMed.
Further Reading
Primary Papers
- Ding F, O'Donnell J, Xu Q, Kang N, Goldman N, Nedergaard M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science. 2016 Apr 29;352(6285):550-5. PubMed.
- Landolt HP, Holst SC. NEUROSCIENCE. Ionic control of sleep and wakefulness. Science. 2016 Apr 29;352(6285):517-8. PubMed.
- Imbach LL, Büchele F, Valko PO, Li T, Maric A, Stover JF, Bassetti CL, Mica L, Werth E, Baumann CR. Sleep-wake disorders persist 18 months after traumatic brain injury but remain underrecognized. Neurology. 2016 Apr 27; PubMed.
- Edlow BL, Lammers GJ. Bringing posttraumatic sleep-wake disorders out of the dark. Neurology. 2016 Apr 27; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Southern California
Both Berezuk et al. and Suzuki et al. focus on the often-neglected role of the Virchow-Robin spaces that control the rate of bulk flow of brain interstitial fluid (ISF), including clearance of potentially toxic substances from brain. Therefore, changes in the volume of these periarterial spaces, as shown by Berezuk et al., in patients with cerebrovascular disorder during sleep, and altered influx of water into the brains of Alzheimer’s disease (AD) patients compared to controls, as shown by Suzuzki et al., are both extremely interesting. The papers illustrate the dynamic physiological changes in the volume of the Virchow-Robin spaces during sleep and disease-driven changes in AD, which both can influence the rate of ISF-CSF solute exchanges and fluid-mediated clearance of solutes from brain.
The broader question remains, however, exactly how changes in the Virchow-Robin space volume contribute to clearance of solutes from brain, and how the size of these perivascular spaces relates to changes in the cerebral vascular system and blood vessels including small vessel disease, loss of blood-brain barrier (BBB) integrity, and transport function, all of which can affect ISF-CSF dynamics. For example, during sleep in the mouse model, it has been shown that accelerated ISF-to-CSF bulk flow rate is responsible for an approximately 40 percent of the increase in total Aβ clearance from the brain, whereas approximately 60 percent increase in Aβ clearance during sleep is regulated by accelerated transvascular Aβ transport (Xie et al., 2013) via transport across the BBB (Deane et al., 2004), as recently reviewed in greater detail (Tarasoff-Conway et al., 2015). However, how these two transport phenomena relate to each other is not currently known. More work is also needed to establish, for example, the consequences of interrupted ISF-CSF flow drainage along the Virchow-Robin spaces when blocked by blood-derived products coming into the brain across the disrupted BBB and/or by proteinaceous products generated in brain, as both processes likely occur simultaneously in many neurodegenerative diseases, including Alzheimer’s.
References:
Berezuk C, Ramirez J, Gao F, Scott CJ, Huroy M, Swartz RH, Murray BJ, Black SE, Boulos MI. Virchow-Robin Spaces: Correlations with Polysomnography-Derived Sleep Parameters. Sleep. 2015 Jun 1;38(6):853-8. PubMed.
Suzuki Y, Nakamura Y, Yamada K, Igarashi H, Kasuga K, Yokoyama Y, Ikeuchi T, Nishizawa M, Kwee IL, Nakada T. Reduced CSF Water Influx in Alzheimer's Disease Supporting the β-Amyloid Clearance Hypothesis. PLoS One. 2015;10(5):e0123708. Epub 2015 May 6 PubMed.
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013 Oct 18;342(6156):373-7. PubMed.
Deane R, Wu Z, Zlokovic BV. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke. 2004 Nov;35(11 Suppl 1):2628-31. PubMed.
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015 Aug;11(8):457-70. Epub 2015 Jul 21 PubMed.
University of Southampton School of Medicine
University of Southampton School of Medicine
Using PET imaging, Suzuki et al. demonstrate that clearance of Aβ from the brain parenchyma into the cerebrospinal fluid is reduced in aged individuals and in those with Alzheimer’s disease (Suzuki et al., 2015). We have demonstrated that clearance of Aβ from the parenchyma occurs along the basement membranes of capillaries and arteries toward the subarachnoid space (Carare et al., 2008; Keable et al., 2016). It is still unclear how much Aβ is released into the subarachnoid space and how much reaches the cervical lymph nodes, although earlier physiological studies estimate that only 10 percent of interstitial fluid reaches the CSF (Carare et al., 2008; Keable et al., 2016; Szentistvanyi et al., 1996). With the accumulation of Aβ in the walls of arteries, the clearance of interstitial fluid along those walls decreases, and thus less Aβ reaches the CSF (Hawkes et al., 2011). Aging also impairs the convective influx of CSF into the parenchyma (Kress et al., 2014). The study by Suzuki et al. provides further evidence that impairment of the different mechanisms for the exchanges between interstitial fluid and cerebrospinal fluid require careful experimental investigation, since the molecular mechanisms may be translated into therapies for Alzheimer’s disease patients.
Impairment of perivascular drainage along the basement membranes of capillaries and arteries results in the accumulation of Aβ in the walls of cortical capillaries and arteries as cerebral amyloid angiopathy, a common finding in Alzheimer’s disease (Iadecola and Zhang, 1996). It is likely that blocking the perivascular drainage pathways in the cortex results in the dilatation of perivascular spaces in the underlying white matter (Weller et al., 2015). The study by Berezuk, Ramirez, and colleagues demonstrates the relationship between sleep and dilated perivascular spaces in a cohort of people of a mean age of 60 years old (Berezuk et al., 2015). The dimensions of the perivascular spaces in the white matter and basal ganglia were correlated to the efficiency of sleep and to the time spent awake after the first sleep and before final awakening. Poor sleep correlated with enlarged perivascular spaces and difficulty in staying asleep correlated especially with the dilated perivascular spaces in the basal ganglia. The anatomical structure of the arteries in the basal ganglia is different from that of the arteries in the cortex, because arteries in the basal ganglia possess a double layer of leptomeninges around them, allowing excess fluid to accumulate between the two (Alcolado et al., 1988; Pollock et al., 1997). Our current observations (manuscript under preparation) demonstrate that arteries in the white matter are also surrounded by a double layer of leptomeninges, in contrast to arteries in the cortex that only have one layer of leptomeninges tightly adjacent to the rest of the wall of the artery.
Sleep is associated with the release of multiple neurotransmitters, including acetylcholine that actively maintains the tone of arteries (Iadecola and Zhang, 1996), contributing to efficient perivascular clearance (Sauvet et al., 2014). Berezuk et al. provide substantial evidence that lack of sleep affects perivascular clearance from the brain parenchyma, probably due to an imbalance in the neurotransmitters that regulate the tone of arteries, resulting in dilatation of perivascular spaces in areas where the anatomical structure of arteries permits the accumulation of excess fluid. This opens the avenue for further experimental studies to clarify the exact mechanisms underlying the dynamics of sleep, neurotransmitters and effects on impaired perivascular clearance.
References:
Alcolado R, Weller RO, Parrish EP, Garrod D. The cranial arachnoid and pia mater in man: anatomical and ultrastructural observations. Neuropathol Appl Neurobiol. 1988 Jan-Feb;14(1):1-17. PubMed.
Berezuk C, Ramirez J, Gao F, Scott CJ, Huroy M, Swartz RH, Murray BJ, Black SE, Boulos MI. Virchow-Robin Spaces: Correlations with Polysomnography-Derived Sleep Parameters. Sleep. 2015 Jun 1;38(6):853-8. PubMed.
Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008 Apr;34(2):131-44. Epub 2008 Jan 16 PubMed.
Hawkes CA, Härtig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, Carare RO. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 2011 Apr;121(4):431-43. Epub 2011 Jan 23 PubMed.
Iadecola C, Zhang F. Permissive and obligatory roles of NO in cerebrovascular responses to hypercapnia and acetylcholine. Am J Physiol. 1996 Oct;271(4 Pt 2):R990-1001. PubMed.
Keable A, Fenna K, Yuen HM, Johnston DA, Smyth NR, Smith C, Al-Shahi Salman R, Samarasekera N, Nicoll JA, Attems J, Kalaria RN, Weller RO, Carare RO. Deposition of amyloid β in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy. Biochim Biophys Acta. 2016 May;1862(5):1037-46. Epub 2015 Aug 29 PubMed.
Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014 Dec;76(6):845-61. Epub 2014 Sep 26 PubMed.
Pollock H, Hutchings M, Weller RO, Zhang ET. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat. 1997 Oct;191 ( Pt 3):337-46. PubMed.
Sauvet F, Florence G, Van Beers P, Drogou C, Lagrume C, Chaumes C, Ciret S, Leftheriotis G, Chennaoui M. Total sleep deprivation alters endothelial function in rats: a nonsympathetic mechanism. Sleep. 2014 Mar 1;37(3):465-73. PubMed.
Suzuki Y, Nakamura Y, Yamada K, Igarashi H, Kasuga K, Yokoyama Y, Ikeuchi T, Nishizawa M, Kwee IL, Nakada T. Reduced CSF Water Influx in Alzheimer's Disease Supporting the β-Amyloid Clearance Hypothesis. PLoS One. 2015;10(5):e0123708. Epub 2015 May 6 PubMed.
Szentistványi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am J Physiol. 1984 Jun;246(6 Pt 2):F835-44. PubMed.
Weller RO, Hawkes CA, Kalaria RN, Werring DJ, Carare RO. White matter changes in dementia: role of impaired drainage of interstitial fluid. Brain Pathol. 2015 Jan;25(1):63-78. PubMed.
Make a Comment
To make a comment you must login or register.