Biogen Antibody Buoyed by Phase 1 Data and Hungry Investors
Quick Links
Last week in Nice, France, a single story took the 12th International Conference on Alzheimer’s and Parkinsons’s Diseases by storm. Everyone who was not on Mars on March 20 probably heard from their local radio or TV station, Twitter, or favorite online source that BIIB037, aka aducanumab, the anti-Aβ antibody developed by the company Biogen in Cambridge, Massachusetts, improved Alzheimer’s symptoms in a large Phase 1 trial. Expectations had been high ever since a Biogen executive told investors at a bank conference in Boston last December that aducanumab was showing a dose-dependent benefit on both amyloid removal and cognition.
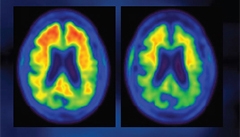
Amyloid PET scan in a BIIB037 trial participant at baseline (left) and after one year on treatment (right). [Image courtesy of Biogen.]
That tease was enough to jack up Biogen’s stock and add billions to its market capitalization prior to the AD/PD conference, which was held March 18 to 22. In Nice on the two days before the aducanumab presentation, prominent Alzheimer’s researchers bantered about how financial analysts were offering them hourly rates up to four figures for comments immediately after the talk. Some analysts were flying in from New York City for the day. During the presentation by Biogen’s Jeffrey Sevigny, one could hear a pin drop in a full auditorium that seats 2,500. The same morning, stories went live online, and the blogosphere and Twitter exploded with chatter. Biogen’s stock price jumped by 7 percent in online trading between Sevigny’s talk at 5:25 a.m. NYC time and the opening bell on Wall Street. It temporarily rose even further before settling there once a heady mix of real results and hyperbole had run its course. In the Nice convention center, some researchers poked fun at the spectacle, waving their arms and shouting: “Sell now! Sell now!” In earnest, however, most scientists were electrified by half of the data Sevigny presented and appropriately cautious about the rest. So what’s the real story? Here is a summary of the data, and what leading researchers think about them.
Formerly called BIIB037, aducanumab is a high-affinity antibody against Aβ. Like its unfortunate cousin bapineuzumab, aducanumab recognizes Aβ’s N-terminal amino acids 3 to 6; however, its slightly wider binding pocket recognizes a structural epitope that is present on aggregated Aβ but absent from monomers. Biogen licensed BIIB037, along with antibodies against aggregated tau and α-synuclein, from the Swiss biotech Neurimmune, picking off some of Neurimmune’s larger panel of antibodies derived from healthy aging donors (for more on the approach, see Apr 2013 conference news).
Aducanumab is notable for its long half-life in human plasma without getting stuck to monomeric plasma Aβ, Neurimmune’s founder Roger Nitsch said at AD/PD. This is important, he said, because it allows aducanumab to accumulate in the brain and find its target there despite antibodies’ generally poor ability to cross the blood-brain barrier.
At AD/PD, Sevigny presented an interim analysis of some of the data of a Phase 1b trial. This was not a final analysis, but merely the first of several data cuts pre-specified in the protocol for this ongoing study, Sevigny emphasized to Alzforum. Clinicaltrials.gov shows this study as collecting data until May 2016.
Following a previous single-ascending-dose study, this larger Phase 1b trial enrolled 166 people whose early Alzheimer’s diagnosis was confirmed by way of a positive scan with the amyloid PET tracer florbetapir. This trial followed a staggered parallel group design, where increasing dose cohorts follow each other. In cohort 1, people were randomized to 1 mg/kg or 3 mg/kg of antibody, each 3:1 to placebo; then an additional cohort was randomized to 10 mg/kg or placebo. Partway into the trial, Biogen followed a recommendation of its data safety-monitoring board and added a third cohort of 6 mg/kg because the highest dose was showing more amyloid-related imaging abnormality, or ARIA-E, a closely watched side effect of anti-amyloid therapy.
This latest cohort has reached its pre-specified first data cut, taken at 30 weeks, but not yet the next cut, which will come at 54 weeks, Sevigny said. Therefore the six-month outcome data he presented at AD/PD compared all doses, but the one-year data did not yet contain the 6 mg/kg cohort. In addition, the trial added a titration cohort in search of ways to minimize the risk of ARIA-E. The placebo-control period of the study involves one injection every four weeks, for a total of 14; participants who complete the one-year blinded portion can choose to add a year of dose-blind extension.
For outcomes, the study set safety and tolerability as the primary, and six-month change in amyloid PET as the secondary endpoints. Exploratory endpoints included this formidable list: one-year amyloid PET; MMSE; CDR-sum of boxes; a version of the NTB neuropsych battery; the FCSRT (which doubled as an inclusion criterion); the NPI-Q neuropsychiatric inventory; and small sub-studies on fluid biomarkers, FDG PET, as well as volumetric, resting-state functional, and ASL spin labeling MRI. On top of that came a quarterly surveillance MRI to monitor ARIA-E.
The groups were well-balanced across arms for baseline characteristics, Sevigny said, with a mean age in the low 70s, a mean MMSE of 25, and mean PET uptake of 1.44 as expressed by composite SUVR. Clinically, about 60 percent were diagnosed as having mild AD; two-thirds carried at least one ApoE4 allele.
At AD/PD, Sevigny showed that amyloid deposition nudged up slightly in the placebo group, but dropped in all treatment groups at week 26 and even more at one year. At six months, this was statistically significant for the 3, 6, 10 mg/kg arms, and at 12 months for the 3 and 10 mg/kg arms. Amyloid was cleared in each of the six cortical regions of interest the scientists measured—frontal, parietal, lateral temporal, sensorimotor, anterior, and posterior cingulate. Importantly, the magnitude of this reduction was far greater than the dips in amyloid load reported in previous trials of bapineuzumab (Rinne et al., 2010) and gantenerumab (Ostrowitzki et al., 2012). In fact, after one year, the 10 mg dose appears to have pulled most amyloid from the brain as that group’s SUVR neared a commonly used threshold of positivity, set here at 1.13. When plotted as amyloid load over time, the groups spread apart by dose, albeit without the results of the 6 mg/kg dose at one year. “Target engagement has been demonstrated,” said Sevigny.
Next, Sevigny showed data for the MMSE and the CDR-SB. The former is a quick screen that taps different domains of cognitive function, the latter is a mixed cognitive/clinical instrument that includes some informant input. On the MMSE, the arms stayed closely together at six months, but by one year they had separated. The placebo group had worsened by 3.1 points, the 1 mg/kg group by about 2 points and the 3 and 10 mg/kg doses by less than 1 point. The two higher-dose groups appeared to stabilize after six months. On the CDR-SB, too, the groups were still together at six months, but by one year they had separated in a dose-dependent way, again with the 6 mg/kg result still pending. On this measure, the placebo group worsened by 2 points and the highest dose by about 0.5 points. The highest dose result was statistically significant and appeared to indicate stabilization. “This supports our hypothesis that amyloid reduction confers a clinical benefit,” Sevigny said.
On safety, Sevigny reported that three patients died, of metastatic cancer, heart attack, and complications from hip surgery, respectively. Two had been on placebo, one in the high-dose group. The study physicians considered the deaths unrelated to aducanumab, Sevigny said. The most common side effect was ARIA-E, a finding of white spots in the magnetic resonance image that reflect vasogenic edema, e.g., fluid in the parenchyma. The surveillance MRIs showed lots of those, mostly in the ApoE4 carriers and at higher doses: one in the 1 mg/kg group, two in the 3 mg/kg group, 10 in the 6 mg/kg group and 13 in the 10 mg/kg group. This was a surprise after the first, smaller trial (see Apr 2013 conference news). Most ARIA-E occurred within the first five doses. Two-thirds were asymptomatic, but one-third caused mild to moderate headaches, visual disturbances, or confusion, which usually resolved within a month, Sevigny said.
“Most participants were unaware that they had ARIA-E,” Sevigny told Alzforum. That is because they did not come in to be seen for symptoms of ARIA-E; rather, their next scheduled MRI picked it up and clinicians then asked if the patients had experienced specific symptoms in the past month. The trial’s protocol guided the study physicians to let patients continue at the same dose, at a lower dose, or to stop dosing, depending on how severe the symptoms were. About half of the participants who developed ARIA-E continued treatment and had no additional ARIA-E; however, one person in the 1 mg/kg group, three in the 6 mg/kg group, and eight in the 10 mg/kg group discontinued treatment. Most were ApoE4 carriers, Sevigny said.
There was little public discussion after Sevigny’s talk, but as soon as he stepped off the lectern, conversation bubbled up and continued for days in the hallways, on the escalators, and over meals. Scientists talked of little else. Initial responses were enthusiastic and became a bit more tempered as time allowed the limitations of this 20-minute presentation to sink in. The Karolinska Institute’s Bengt Winblad from Stockholm spoke for many a battle-weary site leader when he said, “These data for the first time in a long time seem to be positive.” Dennis Selkoe of Brigham and Women’s Hospital in Boston exclaimed, “I am happy! To me a key point is that the amyloid went down at six and even more at 12 months, and that from six months on cognitive decline appears to have been arrested at the highest dose.” Colin Masters of the University of Melbourne in Australia called the data “a game-changer,” and Jeffrey Cummings of the Cleveland Clinic Lou Ruvo Center in Las Vegas noted, “This is the first time we have seen both MMSE and CDR-SB appear to go in the same direction, strongly supported by a biomarker.”
John Hardy at University College London opined, “It was great to see a convincing dose response in every measure. This must give hope to other companies that if you reduce amyloid enough, there will be a clinical benefit.” Enchi Liu of Janssen Research and Development, LLC, agreed. “This is good news for us all. It validates the class of drugs,” she said. Liu worked on bapineuzumab, whose dosing was limited by concerns over ARIA-E; it showed modest target engagement but no efficacy in Phase 3. Many other researchers at AD/PD were similarly relieved at finally getting a shot in the arm after years of setbacks. “This looks good, so long as we remember it’s early days,” said Fred Van Leuven, who co-founded AC Immune, the company that originally developed the Aβ antibody crenezumab and several tau-based immunotherapies. “We are receiving inquiries from people who want to join trials,” said Creighton (Tony) Phelps of the National Institute on Aging in Bethesda, Maryland.
The market’s ebullience will help Biogen finance expensive Phase 3 trials. That said, more than 20 scientists this reporter spoke to were at pains to avoid irrational exuberance. “We have been burned before,” was a common refrain. Most sources drew a dividing line between the strength of the biomarker and safety data, on the one hand, and the limitations of the clinical/cognitive data disclosed thus far.
The target engagement data was widely praised. A large, dose-dependent, and consistent reduction of brain amyloid is a huge step forward, scientists agreed. “They pretty much cleared amyloid out of the brain. That is fantastic, provided it can be repeated,” said Chris Rowe, a PET expert at the University of Melbourne. Besides the SUVR mean value and range for each group, Rowe would like to see the SUVR trajectories of individual participants rendered as spaghetti plots, the way ADNI and AIBL show their data. Amyloid PET data in past cohort studies and clinical trials were marked by considerable variability, particularly when using the brain’s cerebellum as a reference region (see Dec 2014 conference news). Spaghetti graphs show both variation and overall direction at a glance. Also, by depicting each patient’s time course, spaghetti graphs can prove that a few patients did not drive the group result, particularly in the face of a significant number of treatment discontinuations. Other PET experts said the florbetapir data looked cleaner than anything they had ever seen, probably because the drug works so well, but almost to the point where one might begin to worry about a hidden systemic bias. Some scientists wondered if the antibody might bind its epitope so strongly that it edges out florbetapir binding, but Biogen researchers replied they had ruled that out in a competition binding study with Avid Radiopharmaceuticals, the maker of the tracer, before starting the clinical trial.
Of the safety data, the trial’s rate of ARIA-E generated the most debate. Some clinicians thought this side effect was so common at the higher doses that regulatory agencies in some countries might balk at approving a Phase 3 in 2015, insisting on more dose finding or waiting out a first Phase 3 in the United States. Others noted that the field had come a long way in the past decade since ARIA cropped up during the development of bapineuzumab. The key advance now with aducanumab is its strong evidence of target engagement and a clinical signal. Given that, many believe the conversation will evolve from “ARIA is an unacceptable risk” to “How do we best manage ARIA?”
At AD/PD, Janssen’s Liu reinforced this notion. She analyzed bapineuzumab’s Phase 3 dataset to learn if any baseline markers predicted who would develop ARIA-E. No marker did, Liu reported in her talk, but she also found that people who developed ARIA-E subsequently had larger changes in their downstream biomarkers of neurodegeneration, such as CSF tau and brain volume, than those who did not. This strengthens the view that ARIA-E indicates that the antibody is biologically active. The challenge then becomes one of finessing the dose and timing of the injections in such a way that the patient suffers no harm but still gets the benefit. In discussion, some scientists asked if the target engagement and apparent efficacy of aducanumab will spur a change in the paradigm of AD therapy toward the way cancer therapy is viewed. There, the doctor tells the patient: “I will make you very sick before I make you better,” and regulators and society at large accept that.
In all, scientists considered the primary and secondary endpoints of this trial a success. “This trial provides important information about safety and target engagement. This is great medicinal chemistry. They have a dose response and a handle on dosing for the next trial. What more can you ask from a Phase 1b?” said Lon Schneider of the University of Southern California.
A lot more, it appears. At AD/PD, Biogen researchers clearly stated that the trial was not powered for cognitive or clinical outcomes. In fact, Biogen’s trialists had initially designed the study to assess only safety and target engagement, as is customary in Phase 1, but were directed to add exploratory clinical measures. Those are what the investment market and the media latched onto, treating this Phase 1b study as a de facto Phase 2. And Biogen’s leaders in effect did so, too, by deciding to forgo a Phase 2 trial and go straight to Phase 3.
At AD/PD, many scientists pointed out that the study was small. Cognitive outcomes are noisy, and carry little information at fewer than 40 patients per group, said Schneider. As per protocol, Biogen handled the data from these measures by adjusting every person’s actual MMSE and CDR-SB baseline values to a point 0 and scoring change from there together with a p value, rather than plotting the actual values. Some commentators would have preferred to see more raw data of participants’ actual MMSE and CDR-SB values, but others considered this a routine way of presenting such datasets. All agreed the cognitive data was but a preliminary signal.
Others bemoaned that other exploratory outcomes were not presented at AD/PD. When pressed about that, several Biogen scientists assured Alzforum that they simply have not had time yet to analyze those data.
Some pharma scientists considered the decline of the placebo group surprisingly large, given that many patients in the mildest symptomatic stages barely change in a year, especially if they take concomitant medications. However, others disagreed, saying that those expectations come from more heterogeneous cohorts where a proportion has no underlying Alzheimer’s pathology, whereas all participants in this trial did (e.g. Coley et al., 2011).
Some scientists would have liked to see ADAS-cog data to compare aducanumab directly to the crenezumab ABBY trial, which was negative overall but found an efficacy signal in the mild AD subgroup (see Jul 2014 conference news). About half of the patients in the aducanumab trial are at roughly the same mild AD stage. The aducanumab trial did not use the ADAS-cog. In 2013, the Food and Drug Administration issued a guidance for drug development in earlier stages of AD (see Mar 2013 news) that stated that trialists could use a single primary outcome such as the CDR-SB, which includes both cognitive and functional measures. Biogen ran with it, and provided the first evidence that the CDR-SB is sensitive in a treatment study at this stage.
For all outcomes—amyloid removal, cognition and function—scientists puzzled over the treatment discontinuations in the highest dose. Missing data is a common problem in randomized controlled trials. When a disproportionate fraction of a particular group drops out, how the analysis handles that becomes important for the overall result of the trial. The suspicion is that if the worst responders drop out from a treatment but not placebo arm—in this case, for example, the ApoE4 carriers with symptomatic ARIA-E from the high dose—it could make the drug look better than it is. Common ways of analyzing such datasets include:
- Intent-to-treat, aka ITT—data from every randomized person goes into the analysis regardless of whether they received study drug;
- Last observation carried forward, aka LOCF—missing data are imputed based on the last available data from any given patient;
- Per-protocol analysis, where only the data from people who got all treatments and appeared for all visits is included.
Sevigny’s slides, which were not made public but nonetheless circulated as people snapped pictures on their tablet computers, stated in fine print at the bottom that the analysis was based on observed data. Sevigny explained to Alzforum that the aducanumab trial used a modified ITT based strictly on data in hand. That is, it included data from everyone who was randomized, received at least one injection, and underwent at least one set of subsequent assessments, regardless of how many injections they received or whether they completed all visits. People who discontinued treatment were urged to come for their scheduled assessments anyway, and those data went into the analysis. In other words, treatment discontinuation is not the same as dropout. When someone skipped a visit or dropped out for good, missing data were not imputed, Sevigny said. So hypothetically, the data from a person who received four injections, developed symptomatic ARIA-E, discontinued treatment, but came for his or her 54-week assessment, would all go into the analysis. Likewise, the data from a participant who failed to show up for his or her 54-week visit would simply be missing from the pool, rather than estimated based on his or her prior trajectory.
All in all, the scientists were immensely pleased about the results for which the study was powered, and cautious about the rest. “Let’s not overvalue the clinical outcomes. Biogen was careful to say they are exploratory. The rest of the world seems to think we have to make inferences on the clinical outcomes as they were presented. We will be able to assess the clinical outcomes soon enough in the Phase 3 trials,” said Schneider. By March 24, analysts on Twitter had calculated aducanumab’s potential worth at $90 billion.—Gabrielle Strobel
References
Therapeutics Citations
News Citations
- Safe at 4 Grams? No ARIA at High Dose of Human Aβ Antibody
- Immunotherapy I: Baby Steps, but No Breakthroughs
- Crenezumab Disappoints in Phase 2, Researchers Remain Hopeful
- FDA Invites Comment on Drug Testing Guidance for Early AD
Paper Citations
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, Rodriguez Martinez de Liano S, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010 Apr;9(4):363-72. Epub 2010 Feb 26 PubMed.
- Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, Klunk WE, Ashford E, Yoo K, Xu ZX, Loetscher H, Santarelli L. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012 Feb;69(2):198-207. Epub 2011 Oct 10 PubMed.
- Coley N, Andrieu S, Jaros M, Weiner M, Cedarbaum J, Vellas B. Suitability of the Clinical Dementia Rating-Sum of Boxes as a single primary endpoint for Alzheimer's disease trials. Alzheimers Dement. 2011 Nov;7(6):602-610.e2. Epub 2011 Jul 13 PubMed.
Other Citations
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.