From Natural History, A "Renaissance" for Amyloid Hypothesis
Quick Links
Read a PDF of the entire series.
Italy’s beautiful city of Florence was the cradle of the Renaissance, a period of rebirth for the arts and commerce that helped Europe shed the comparative crudeness and deprivation of the Middle Ages. It was perhaps inevitable, then, that scientists quipped about Florence as a fitting setting for what they perceive to be a resurgence of the amyloid hypothesis after a beleaguered period of setbacks. On 9 March at the 11th International Conference on Alzheimer’s and Parkinson’s Diseases AD/PD 2013, a plenary session titled “The Amyloid Cascade and Alzheimer’s Disease: Hypothesis or Theory?” filled the venue’s largest auditorium to capacity. Leading scientists recounted why, to their minds, the hypothesis can now be called a theory and is poised on the brink of a more definitive test in the clinic. Some in the audience came away inspired, citing genuine passion on the part of the speakers. Others noted that Lilly’s sponsorship of the symposium—with its slick pocket folder handouts and the pharma company’s oversized logo emblazoned beside the speakers—gave the session an overtly commercial sheen.
In other sessions at AD/PD 2013, Lilly scientists claimed that solanezumab’s small but independently verified Phase 3 benefit for cognition in people with mild AD (see ARF AAN news story) constituted provisional validation of the amyloid hypothesis—a statement that reliably provokes controversy. At the same time, there was a sense at the conference that natural history data, such as those emerging from large groups of people monitored for biomarker change over many years, are swaying the debate in favor of the amyloid hypothesis. The plenary featured a major such paper, published March 7 in Lancet Neurology online from the Australian Imaging Biomarkers and Lifestyle Study of Ageing (AIBL; see Villemagne et al., 2013). AIBL collects clinical, cognitive, neuroimaging, lifestyle, and biomarker data at each visit. “The only valid way of testing the amyloid hypothesis is to understand the natural history of Alzheimer’s disease,” Colin Masters at the University of Melbourne said at the plenary.
In that paper, AIBL researchers describe how they have taken three or more successive measurements, spaced three to five years apart, in 200 AIBL participants across the spectrum of normal cognition to Alzheimer’s dementia. Of the 200 participants analyzed for this paper, 72 had four assessments, and 12 had five assessments covering six years. From those data, the scientists worked out the rate by which amyloid deposition evolves over time and in relation to changes in brain atrophy and cognition. This is the largest longitudinal dataset on how the main manifestations of Alzheimer’s disease change over time before dementia is fully expressed. These data test a proposed staging diagram. AIBL has been running for eight years and is currently entering all 72-month data to refine the rate calculations obtained in the current paper, Masters said.
What did the researchers find? They divided the participants into those who accumulated Aβ over the years and those who do not. Among the "accumulators," cognitively normal people deposit the most amyloid, at a rate of 2.6 percent per year; people with MCI deposit at 2 percent per year; and those with AD at 1 percent per year. The researchers connected the dots by transforming these three- to five-year stretches of true data along the entire spectrum into a cumulative kinetic over time. They found that it takes 12 years on average for a person who is free of amyloid but starting to accumulate it to reach the threshold of positivity in brain amyloid PET, set here as an SUVR of 1.5. Once there, it takes the average person another 19.2 years to have developed Alzheimer’s dementia, defined here as a clinical dementia rating (CDR) of 1.0. “We found the full evolution of this illness takes 30 years,” Masters told the audience.
Importantly, the other markers AIBL tracks—hippocampal and gray matter volume, episodic memory, and other cognitive functions—change much later and faster, within five years prior to this same CDR anchor point.
Sequence of change in the decades-long preclinical development of Alzheimer’s dementia. Image courtesy of Villemagne et al., Lancet Neurology 2013
Since the paper was written, AIBL scientists, including Victor Villemagne, Chris Rowe, and others in Australia, have bulked up the dataset underpinning these timelines. They have added 300 healthy or MCI participants to the imaging cohort in AIBL and another 300 in affiliated studies such as the AIBL-Women's Healthy Aging Study, and the AIBL Veterans Study. They also intend to scan new cohorts for preclinical AD trials to include 300 people. Once more longitudinal scan data are analyzed, “The predictive validity of this curve will become patently obvious,” Masters said.
Other natural history studies are finding much the same thing, though none have as much longitudinal amyloid PET as AIBL. On 5 March 2013, U.S. researchers published similar rates based on two or more serial assessments in 260 participants in the Mayo Study Clinic of Aging (Jack et al., 2013). The studies differ in details, such as how they set their measurement cutoffs, but they converge in their main findings.
Both studies report that for about 15-17 years onward from when a cognitively normal person becomes amyloid positive, the amyloid deposition curve is quite linear, a feature that makes measuring drug effects easier. Also at this AD/PD plenary, John Morris of Washington University, St. Louis, Missouri, pointed to the full decade that passes between when a person deposits significant brain amyloid and when subsequent markers of neurodegeneration and then cognition begin to change. This suggests that in this period of amyloid deposition without serious disruption of synaptic integrity, an anti-amyloid monotherapy may work, but after that, “We may need to use drugs in combination that target more than one mechanism.”
The kinetics of brain Aβ deposition fit a sigmoid curve, with a long, linear stretch in the middle. Image courtesy of Villemagne et al., Lancet Neurology 2013
Both AIBL and the Mayo studies also find that above a certain level of deposition, expressed here as an amyloid PET SUVR of roughly 2.2, the slope levels off and later even becomes biphasic; in other words, the curve dips a bit as dementia advances. In toto, the serial data available to date add up to a sigmoid curve with a long, drawn-out ascent prior to and into the MCI/prodromal stage of Alzheimer’s disease. ADNI finds similar curves, but fewer participants have had serial scans with the same PET tracer to enable calculation of longitudinal rates. ADNI 2 and 3 will generate such data. The first published attempts at quantitatively characterizing longitudinal amyloid deposition, starting from smaller groups and fewer scans, came from John Morris, Mark Mintun, and colleagues at Washington University (Vlassenko et al., 2011; Vlassenko et al., 2012).
Other large natural history initiatives are currently collecting and analyzing serial data on autosomal-dominant forms of Alzheimer’s disease. They have published cross-sectional data of the baseline examination of people at different ages and plotted it to depict extrapolated change over time. These data, too, converge in large part with the true longitudinal data from AIBL, Masters told the audience. In particular, change in Aβ deposition and memory correlate closely between the Dominantly Inherited Alzheimer Network (DIAN) and the AIBL (see also ARF related news story). Curiously, hippocampal atrophy at present looks different, where DIAN appears to find changes earlier than AIBL. “Whether that difference is technical or biological must be worked out,” Masters said.
The data available thus far from DIAN have confirmed that autosomal-dominant and late-onset AD are closely similar in terms of their biomarker and clinical course, Morris said at the plenary. The major phenotypic differences are that motor symptoms, seizures, and amyloid deposition in the basal ganglia occur more often in autosomal-dominant AD than in LOAD (for detailed coverage of DIAN, see ARF related news series). Even as DIAN is continuing its natural history study, with 304 people age 19 and up enrolled and new centers joining, the network has also pushed clinical trials toward an ultimate goal of combination treatments to halt the disease. “DIAN data show that the disease first manifests in overproduction of Aβ. This supports the amyloid hypothesis, but the reason we are doing this is not only to understand the natural history of the disease, but also to test it directly,” Morris said.
To that end, the network created a trials unit (DIAN TU), led by Randall Bateman at WashU. This precompetitive group of 10 pharma companies collaborates on trial planning and nominates their drugs for DIAN trials. Two members, Roche and Lilly, joined a competitive investigation of their unapproved therapies side by side in a single trial, the first such instance in Alzheimer’s disease drug development. Roche and Lilly worked out a contract with WashU in less than a year, agreed on a single protocol for an anti-amyloid preclinical treatment trial, and obtained FDA approval without any protocol changes, as well as IRB approval. “It took less than three months from the announcement of the three selected drugs to our first trial participant signing informed consent on December 31, 2012,” Morris said. That participant subsequently was randomized to receive active drug or placebo on 18 March 2013, officially inaugurating the era of secondary prevention trials in AD, said Morris.
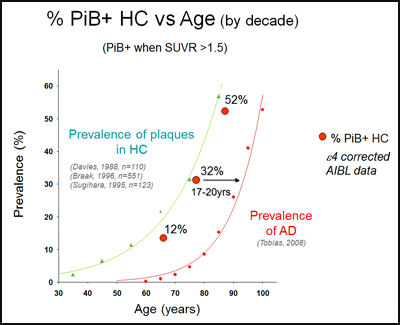
At the AD/PD symposium, Reisa Sperling of Brigham and Women’s Hospital in Boston first noted that many studies around the world are supporting the amyloid hypothesis by suggesting that brain amyloid positivity increases a person’s risk of cognitive decline. AIBL’s March 7 paper demonstrates that by chronicling accelerating decline in people who accumulate brain amyloid but not in those who don’t. The more amyloid a person already has in the brain, the faster this happens. Other studies, such as Sperling’s own Harvard Brain Aging Study, are finding that cognitively normal people who were originally recruited merely to serve as a comparison to the AD groups are turning out to be most interesting. They show amyloid positivity in the same age-dependent fractions that prior pathology studies would have predicted. These amyloid-positive "controls" are now showing subtle cognitive decline in serial assessments. Their age distribution precedes the demographic prevalence of Alzheimer’s dementia by about 17 years. This offers an opportunity for clinical intervention to prevent them from reaching that stage. “We have a 15-year window to act, so I see a glass half full,” Sperling said.
Can therapies prevent red dots from turning into diamonds? AIBL PET data in cognitively normal people show the same age distribution for having amyloid in the brain (red dots) as prior postmortem series (green triangles). This precedes the age distribution for having Alzheimer’s dementia (red diamonds) by about 18 years. Image courtesy of AIBL
But while natural history data—and also genetics (see below)—are increasingly incontrovertible, upcoming clinical trials of the amyloid hypothesis are still groping in the dark on some important questions. That is, in part, because the field remains far from consensus about which toxic species to target, how much amyloid lowering might be right, and when it should be done. Alzforum has closely covered the calls for earlier, preclinical-stage trials prior to extensive neurodegeneration, as well as a budding movement toward combination trials (see ARF A4 news story; see ARF combination trials news series). In Florence, Sperling emphasized that trials need better synaptic markers. For example, in the Harvard Brain Aging Study, functional MRI shows evidence of disconnection or dysfunction of critical networks in amyloid-positive but cognitively normal people; also, their cortex subtly thins out in the precuneus, the posterior cingulate, and other areas that have more pronounced changes later on. These people do worse (Sperling et al., 2013) and decline faster in their thinking than those without amyloid (Lim et al., 2012). For its part, ADNI shows that amyloid-positive cognitive normal people already suffer a slow and subtle cognitive decline (Landau et al., 2012). AIBL shows this, too. “This kind of finding will come to redefine what is normal aging,” Sperling said.
All these studies have large error bars, however. The variation stems in part from cognitive reserve, whereby education and cognitive engagement allow people to withstand the effects of amyloid in their brains for some time. And it stems partly from modifying genes that determine how resilient a person is to Alzheimer’s.
What does genetics say on the topic of the amyloid hypothesis, anyway? Giving a playful nod to Florence, John Hardy from University College London, U.K., borrowed a quip from a book on Leonardo da Vinci: “You can say that genetics has delivered a Dimostrazione for the amyloid hypothesis.” Hardy, whose group discovered the London pathogenic APP mutation and later mutations in tau, α-synuclein, and TREM2, is widely cited for having co-articulated the amyloid hypothesis of amyloid production and downstream tangle pathology (e.g., Hardy and Selkoe, 2002), even though historically, George Glenner first proposed an amyloid-only version of the hypothesis; see Glenner and Wong, 1984. Alas, in subsequent years, Hardy publicly voiced doubt about its explanatory power amid concern that the genetics of late-onset AD failed to adequately support the hypothesis while molecular biology was unable to elucidate the primary function of APP. Those years of uncertainty have now given way to new confidence fueled by technical advances in genetics. In the last few years, GWAS have identified low-risk common variants, exome sequencing has begun turning up high-risk variants, and several large whole-genome sequencing projects are underway.
Importantly, all the genes that GWAS have identified map onto the amyloid hypothesis, said Hardy. They may not affect Aβ levels directly—in fact, in a separate talk at AD/PD 2013, Lawrence Rajendran of the University of Zurich reported that they do not. Yet they fall into the pathways of endosome vesicle recycling, cholesterol metabolism, and innate immunity, all of which intersect with Aβ homeostasis in the complex pathophysiology of AD. For example, the gene TREM2, found last year, reacts to amyloid plaques with enhanced expression, and its protein functions to keep activated microglia in a beneficial phagocytic state, Hardy said. That microglia engulf amyloid has been shown by other groups many years ago, but human genetics validates the relevance of this process in Alzheimer’s disease.
The genetics of Alzheimer’s remains incomplete until new techniques will have accounted for the entire genetic burden of the disease. Even so, the lessons today are that the Mendelian pathogenic Alzheimer’s mutations are all involved in Aβ production, whereas a protective variant cuts the opposite way. GWAS and exome sequencing variants map to defined pathways that are consistent with the amyloid cascade hypothesis. “I feel bullish that we can start to call the hypothesis a theory,” Hardy said.
In discussion with the audience, Sperling pointed out that preventive treatment works in cancer, HIV/AIDS, stroke, osteoporosis, diabetes, and heart disease. She recalled the cholesterol wars, where raging debate about good versus bad cholesterol did not stop the field from running secondary prevention trials in familial hypercholesterolemia (as do DIAN and API), and then thousand-person studies in people thought to be at risk (as does A4). Reduction of cholesterol is now estimated to have reduced cardiac mortality by nearly a third. “For this to work, amyloid does not have to be the cause; it just has to be a critical factor in the cascade,” Sperling said. “We should stop arguing about whether it is amyloid or tau. It is both and it is also other factors that we have not discovered yet. As 10,000 baby boomers are turning 65 every day in the U.S., we need to target everything—amyloid, tau, neuroprotection, metabolism, and the innate immune system.”—Gabrielle Strobel
References
News Citations
- Anti-Amyloid Results Show Modest Benefits, Mild Side Effects
- Expanding the Network, DIAN Starts Showing Longitudinal Data
- DIAN Trial Picks Gantenerumab, Solanezumab, Maybe BACE Inhibitor
Paper Citations
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins R, Maruff P, Ames D, Rowe CC, Masters CL, Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013 Apr;12(4):357-67. Epub 2013 Mar 8 PubMed.
- Jack CR, Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, Pankratz VS, Senjem ML, Gunter JL, Mielke MM, Lowe VJ, Boeve BF, Petersen RC. Brain β-amyloid load approaches a plateau. Neurology. 2013 Mar 5;80(10):890-6. PubMed.
- Vlassenko AG, Mintun MA, Xiong C, Sheline YI, Goate AM, Benzinger TL, Morris JC. Amyloid-beta plaque growth in cognitively normal adults: longitudinal [11C]Pittsburgh compound B data. Ann Neurol. 2011 Nov;70(5):857-61. PubMed.
- Vlassenko A, Blazey T, Su Y, Xiong C, Goate A, Benzinger T, Mintun M, Morris J. Conversion to Preclinical Alzheimer’s Disease in Cognitively Normal Adults: The Characteristics from Longitudinal [11C] PIB PET Study. Human Amyloid Imaging Abstract. 2012 Jan 1;
- Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS, Sabbagh MN, Sadowsky CH, Carpenter A, Davis MD, Lu M, Flitter M, Joshi AD, Clark CM, Grundman M, Mintun MA, Skovronsky DM, Pontecorvo MJ, . Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol Aging. 2013 Mar;34(3):822-31. PubMed.
- Lim YY, Ellis KA, Pietrzak RH, Ames D, Darby D, Harrington K, Martins RN, Masters CL, Rowe C, Savage G, Szoeke C, Villemagne VL, Maruff P, . Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012 Oct 16;79(16):1645-52. PubMed.
- Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, Weiner MW, Jagust WJ, . Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012 Oct;72(4):578-86. PubMed.
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002 Jul 19;297(5580):353-6. PubMed.
- Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131-5. PubMed.
Other Citations
External Citations
Further Reading
News
- Expanding the Network, DIAN Starts Showing Longitudinal Data
- DIAN Trial Picks Gantenerumab, Solanezumab, Maybe BACE Inhibitor
- NIH Funds Four Clinical Trials in ADCS Renewal
- Tau, α-Synuclein Spread: Crazy Stuff—How Might It Work?
- Like Star Born of Supernova, Plaque Born of Exploded Neuron?
- Can Dousing PyroGlu-Aβ Treat Alzheimer’s Disease?
- Field Ramps Up "Mini" Mouse MRI
- LRRK Watchers’ Eyes Turn to Inflammation, Autophagy, Kinase
- Can Cancer Therapy Be Neurodegenerative Wonder Drug?
- Taking Aim at M1: Old Hat or New Target?
- Dementia in Movement Disorders: What Causes It?
- BACE Inhibitors Barrel Forward—Next Hurdles: Safety, Efficacy
- Anti-Amyloid Results Show Modest Benefits, Mild Side Effects
- In Pursuit of Toxic Tau
- Sleep Patterns, Circadian Clock Linked to Aβ Oxidative Stress
- Combination Drug Trials: Time to Open a New Front in AD?
Annotate
To make an annotation you must Login or Register.



Comments
No Available Comments
Make a Comment
To make a comment you must login or register.