Exosomes: The FedEx of the Nervous System?
Quick Links
See Part 6 and Part 7 of this series.
Move over, synaptic vesicles. Scientists are starting to appreciate that cells of the nervous system transmit information in a different type of vessel, as well. At the Society for Neuroscience annual meeting, held November 15 to 19 in Washington, D.C., researchers discussed the latest on exosomes—tiny bundles of cellular material that pass among cells. It appears that neuroglial cells in the brain use them like care packages. Astrocytes pack exosomes with specific messenger RNAs and then dispatch them to neurons, which soak them up and express the transcripts. However, some of the packages are bad news. Gioblastomas use exosomes to suppress the brain’s immune system, allowing the tumor to run rife.
“The neuroscience community is just catching on to this relatively novel form of communication," said Eva-Maria Krämer-Albers, Johannes Gutenberg University in Mainz, Germany. "Exosomes will completely change our view of how cells in the nervous system interact with each other,” she predicted. This basic biology could turn out to be important in Alzheimer's and other neurodegenerative diseases, as evidence has emerged that exosomes can transport and may even make toxic peptides such as Aβ and α-synuclein. (See Part 6 of this series.)
Exosome Primer—Vesicles Within Vesicles
All cells of the nervous system secrete exosomes. These small, 50-100 nm vesicles form when the membrane of a late endosome invaginates, then pinches off to make a small vesicle within the lumen of the endosome. This can occur many times, turning the endosome into a multivesicular body. These merge with lysosomes, spilling the vesicles into an acidic environment, which degrades them. However, if a multivesicular body instead fuses with the plasma membrane, the intraluminal vesicles escape into the extracellular space as exosomes (see image below). Exosomes may contain any of the molecules found in endosomes, including proteins and lipids, even RNAs specific to the cell that released them. Exosomes rid the cell of debris, but not all their cargo goes to waste. With the discovery that exosomes contain mRNA (see Valadi et al., 2007), scientists realized that these vesicles also could transfer information between cells in the nervous system.
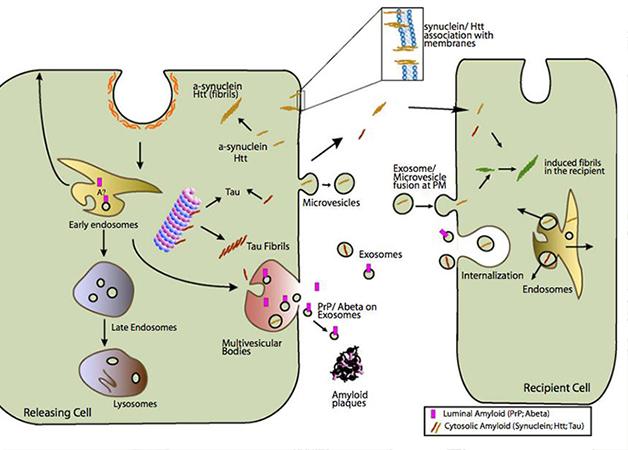
Enter the Exosome: Multivesicular bodies (pink) that merge with the cell’s plasma membrane squirt exosomes (small gray-green circles), which may release toxic proteins such as Aβ and α-synuclein into the extracellular environment. [Image courtesy of Aguzzi and Rajendran, 2009, Neuron.]
A Regenerative Boost to Peripheral Neurons …
Researchers are trying to figure out which of the components exosomes carry are key for their communication. Felipe Court, Pontifica Universidad Católica, Santiago, Chile, studies how exosomes convey messages from Schwann cells to axons. These glial cells cozy up to mature axons in the peripheral nervous system and wrap myelin sheaths around them. Schwann cells inhibit the further growth of axons unless axons sustain damage, in which case Schwann cells undergo a sort of reprogramming. They then release exosomes that stimulate axonal regeneration when taken up by nearby sensory neurons in vitro, (see Lopez-Verilli et al., 2013). Just what in those exosomes might trigger the axons? Court suggested it was RNA.
Knowing that exosomes contain messenger RNA, scientists led by Court used high-throughput sequencing to identify what kinds of transcripts might be in exosomes from Schwann cells. The exosomes were rich in mRNAs that encode proteins involved in the assembly, organization, and regeneration of cells, as well as in axon growth and regeneration. This mRNA profile looked completely different from that of the original Schwann cells. “That’s very interesting because it means that the cell isn’t just putting RNA randomly into the vesicles, but is packaging specific transcripts for export,” Court said. Schwann cells concentrated the mRNA for neurofilament H, for instance, in their exosomes. Schwann cells do not actually make this protein. “This means that the cells are making transcripts, putting them in exosomes, and exporting them just for neurons to use,” said Court.
The exosomes also contained specific micro RNAs (miRNAs) that seemed to lend axons a helping hand. Out of 200 genes Court and colleagues identified as being involved in axonal regeneration and growth, in-silico analysis flagged 120 of them as targets of the particular miRNAs found in Schwann cell exosomes.
To find out how these exosomes affect axons, the group performed tests on cultured sensory neurons from the rat dorsal root ganglion. Isolating these neurons damages their axons, which begin to regenerate two to three days later. However, if the researchers added exosomes from Schwann cells to the sensory neurons, the axons began to regenerate immediately. The effect seemed unique to Schwann cell exosomes, as those from astrocytes did nothing to enhance axon growth.
Looking at the sensory neurons just a day after culturing, the researchers found a unique miRNA profile in the neurons treated with exosomes. Many miRNAs that predominated in the untreated neurons were absent in the treated ones. In fact, the miRNA profile of the latter resembled that of sensory neurons that had been damaged during isolation and were actively regenerating in culture. “Schwann cell exosomes are activating a specific set of genes [in the neurons],” said Court. In the future, researchers may be able to use exosomes to reveal new genes involved in axon regeneration, he said.
… and to Neurons in the Brain
In the same way that Schwann cells deliver exosomes to aid neurons in the periphery, recent studies suggest that oligodendrocytes, their central nervous system equivalents, shower exosomes onto nearby neurons. Krämer-Albers previously discovered that mouse oligodendrocytes secrete exosomes in response to synaptic activity in adjacent neurons (see Frühbeis et al., 2013). At SfN, she reported that in vitro, mouse cortical neurons pretreated with exosomes from oligodendrocytes turned on cell-survival pathways and resisted nutrient deprivation, oxidative stress, and ischemia. They also fired more often (see Fröhlich et al., 2014). The exosomes did not affect axon growth, however, nor did they protect neurons if they were applied after the stressor. “Unlike exosomes from Schwann cells, these don't seem to have regenerative potential, but rather, are neuroprotective,” Krämer-Albers said.
That's not to say that oligodendrocyte exosomes don't support axons. The German scientists found that, in mouse hippocampal neurons, the exosomes sped up anterograde transport of cargo down axons and cut down on the number of traffic stoppages. This suggests that the oligodendrocytes run a kind of “delivery” service, whereby neurons call on the helper cells to release neuroprotective care packages. “This may be a mechanism to maintain the long-term integrity of axons, which stretch far away from the nourishing cell body,” said Krämer-Albers.
Despite the growth of in vitro data on exosomes, scientists have yet to demonstrate that they work the same way in vivo. “It will be extraordinarily challenging to validate these findings in the human brain,” David Brody, Washington University in St. Louis, wrote to Alzforum. Krämer-Albers agreed, noting that the vast majority of exosome studies have relied on cultured cells because scientists lack the tools to manipulate exosome release in animals. She said the physiological role of exosomes may remain in question until the field comes up with specific ways to target their secretion and transfer in vivo.
Some researchers have come close. In a symposium, Vivian Budnik, University of Massachusetts Medical School, Worcester, reported that exosomes signal between pre- and postsynaptic cells at the neuromuscular junction in the fly brain (see Korkut et al., 2013). Those signals are crucial to maintain synaptic plasticity. She also found that Wnt signals important for fly brain development pass between neurons via exosomes (see Koles et al., 2012). “Though exosome biology is likely conserved through evolution, we still lack evidence for its function in the mammalian brain,” Krämer-Albers told Alzforum.
The Dark Side of Exosomes
Most of these findings put a positive spin on exosome signaling, but exosomes may not always be good for the brain. Tumors seem to exploit them to fuel their growth. One lethal brain tumor, glioblastoma multiforme, arises from astrocytes or their precursors. “We don’t know how these tumors are so aggressive, but we think the vesicles they release are priming the environment around them to allow them to progress more rapidly,” said Xandra Breakefield, Massachusetts General Hospital, Charlestown. These extracellular vesicles include exosomes and microvesicles released directly from the cell membrane (see image above). Researchers know that they help tumors develop new blood vessels and suppress immune cells that might rein in the cancers (see Iero et al., 2008 ). The RNA profile inside these vesicles differs starkly from that of the original tumor, and the cells that take up the vesicles translate the RNAs within them (see Skog et al., 2008).
Recently, researchers at Breakefield’s lab found that micro RNAs miR-451 and miR-21 are enriched in extracellular vesicles from glioblastoma multiforme cells and modify nearby microglia. While cultured microglia usually contain small amounts of these miRNAs, the cells rapidly took up gliobastoma exosomes, raising miR-451 and miR-21 levels 10- to 40-fold. Both these miRNAs target the messenger RNA for the transcription factor c-Myc. C-Myc expression fell in microglial cultures exposed to tumor vesicles. Breakefield is unsure how downregulation of c-Myc would modify tumor growth, but suggested that it may subdue the immune system, giving tumor cells free rein to grow. In a mouse glioblastoma model, microglia appeared to be attracted to the tumors. They took up tumor-derived vesicles, bumping up miR-451 and miR-21, while c-Myc expression fell.
Overall, the picture emerging is that extracellular vesicles transfer information through gene expression and induction of new properties in nearby cells, said Lawrence Rajendran, University of Zurich, who co-chaired the session. It remains to be seen how the RNAs are targeted to vesicles, how cells take them up, and how molecules get out of the exosomes once they enter the new cell, he added. “On top of that, the roles exosomes play in physiology and pathology are still to be determined,” he said.
Other researchers who heard the talks were intrigued. “The data are very convincing that exosomes are not just a way for the cell to dispose of things, but also to pass messages to other cells,” said Dimitrios Kapogiannis, National Institute on Aging, Baltimore. “I predict that exosomes will be an even hotter topic in the near future because more and more people are researching their biological function.” Scientists were particularly excited by the possibility that exosomes may propagate, and even generate, pathogenic proteins involved in neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases (see Part 6 of this series).—Gwyneth Dickey Zakaib.
References
News Citations
- Exosomes: Purveyors of Neurodegenerative Disease?
- Hats Off to ApoE—Key to Formation of Functional Amyloid
Paper Citations
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007 Jun;9(6):654-9. Epub 2007 May 7 PubMed.
- Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013 Nov;61(11):1795-806. Epub 2013 Aug 30 PubMed.
- Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Möbius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Krämer-Albers EM. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013 Jul;11(7):e1001604. Epub 2013 Jul 9 PubMed.
- Fröhlich D, Kuo WP, Frühbeis C, Sun JJ, Zehendner CM, Luhmann HJ, Pinto S, Toedling J, Trotter J, Krämer-Albers EM. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci. 2014 Sep 26;369(1652) PubMed.
- Korkut C, Li Y, Koles K, Brewer C, Ashley J, Yoshihara M, Budnik V. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013 Mar 20;77(6):1039-46. PubMed.
- Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, Budnik V. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012 May 11;287(20):16820-34. Epub 2012 Mar 21 PubMed.
- Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008 Jan;15(1):80-8. Epub 2007 Oct 12 PubMed.
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008 Dec;10(12):1470-6. Epub 2008 Nov 16 PubMed.
Further Reading
Papers
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010 May 19;30(20):6838-51. PubMed.
- Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3:24722. Epub 2014 Nov 13 PubMed.
Primary Papers
- Rajendran L, Bali J, Barr MM, Court FA, Krämer-Albers EM, Picou F, Raposo G, van der Vos KE, van Niel G, Wang J, Breakefield XO. Emerging roles of extracellular vesicles in the nervous system. J Neurosci. 2014 Nov 12;34(46):15482-9. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.