Field Ramps Up "Mini" Mouse MRI
Quick Links
Read a PDF of the entire series.
As magnetic resonance imaging (MRI) resolution improves and more animal models become available, scientists are stepping up their use of mouse MRI. But wait a second—the field has already developed MRI methods for humans; why advance them in mice? At the 11th International AD/PD Conference held 6-10 March 2013 in Florence, Italy, presenters made their case. Longitudinal MRI studies in mice, which aim to track disease progression over time, will help translate findings from animal to human, they explained.
“Not only will we understand our models much better, but we will be able to better see the effects of therapies in mice,” said Nick Fox, University College London, U.K. For example, in immunotherapy, the MRI response of Alzheimer’s patients—shrinkage in people who appear to respond to the vaccine or antibody—has surprised researchers, and having this outcome measure available in mice would enable a more thorough understanding of it.
Researchers use MRI techniques in humans to image brain characteristics such as anatomy in structural MRI, oxygen consumption in functional MRI (fMRI), and white matter integrity in diffusion tensor imaging (DTI). All of these approaches are currently used to follow how disease worsens in people. The problem is that scientists use different methods to understand the course of disease in mice. For instance, they may sacrifice animals at multiple time intervals to measure amyloid or tau pathology. Such methods are variable, costly, and require large numbers of mice. By developing non-invasive MRI technology for mice, scientists can measure disease in the same mouse, compare results between animals and people, and use fewer animals in research. “Instead of getting just a snapshot of a process, you can follow it over the whole disease course,” said Jan Klohs, University of Zurich, Switzerland.
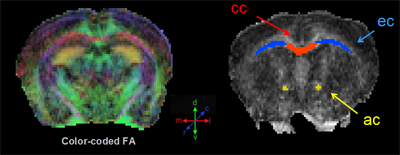
Mouse Brain MRI
Color-coded fractional anisotropy map (left); manually drawn regions of interest (right) include the corpus callosum, external capsule, anterior commissure. Image courtesy of Moira Marizzoni
Which aspects of disease are amenable to modeling in this way? Gianluigi Forloni, Mario Negri Institute of Pharmacological Research, Milan, Italy, presented data that highlight atrophy of some brain regions in Alzheimer’s disease animal models. He and colleagues compared structural MRI results between wild-type mice and single (PDAPP), double (APP/PS1), or triple (TauPS2APP; Grueninger et al., 2010) transgenics. Over time, the volume of the putamen shrank in all three mutants relative to controls, suggesting it could be a good disease marker in these mice. “That was a surprise because we expected to see an effect in the cortex and in the hippocampus, where there is more accumulation of β amyloid,” Forloni told Alzforum.
Why the putamen, wondered audience members? This is a brain area affected in Parkinson’s disease. The authors are not sure, but researchers see abundant striatal plaque deposition in humans with familial AD, which these mice model, said Forloni. The putamen is part of the striatum. In fact, amyloid PET retention in familial AD frequently begins in the striatum (ARF related news story). Forloni’s group next plans to investigate whether the mice’s striatal atrophy is due to cell loss. In the future, they may use this marker to see how drug treatments affect this volumetric decline.
Mice may also model white matter damage, said Moira Marizzoni, of IRCCS Fatebenefratelli in Brescia, Italy. She performed a longitudinal DTI study on single (PDAPP), double (TASTPM; Howlett et al., 2004), and triple (TauPS2APP) transgenic mice to find biomarker differences from wild-type. Several markers of white matter distinguished double transgenic mice from controls. Marizzoni found a 10-15 percent change in fractional anisotropy, radial diffusivity, and axonal diffusivity mainly in the corpus callosum and anterior commissure, and less in the cerebral peduncle. Human AD patients have similar white matter changes in the corpus callosum (see Acosta-Cabronero et al., 2012). These results suggest that, once validated, these biomarkers could be promising markers of disease progression and drug effects on white matter damage in mice, Marizzoni believes. However, the single and triple transgenic mice did not show these white matter changes.
Mice can model functional connectivity, suggested Joanes Grandjean, ETH Zürich, Switzerland, in a poster presentation. Using resting state fMRI in the ArcAβ mouse model, Grandjean found that the sensory and motor cortices in the transgenics were less correlated with their respective contralateral sides than in wild-type. These differences showed up when mice were just five months old, two months before plaque deposited. Because of concerns that the anesthesia required to keep mice still in the scanner could interfere with functional connectively, researchers are moving toward doing such studies in more alert animals, he told Alzforum.
Another advantage of developing these MRI techniques in mice is that single pathologies can be observed and specifically targeted for intervention, without the comorbidities and mixed forms of dementia that complicate the picture in humans, Grandjean said. “This allows for more homogeneous groups and requires a smaller sample size than needed for human studies.”
Technological improvements have been a boon to mouse MRI, said Manfred Windisch of the CRO QPS, formerly JSW, in Graz, Austria. Stronger magnets grant higher resolution to visualize minute structures in the mouse brain, and special hardware and software reduce the signal-to-noise ratio. “You can collect a vast number of data in a single mouse, and get broader information about a drug’s effect,” he told Alzforum. Treatment studies in Alzheimer’s would benefit particularly from amyloid PET in mice, but despite one reported technical improvement (see ARF related news story), mouse amyloid PET is still not a viable tool.
On MRI, too, the researchers will hopefully go further with their data, said Menahem Segal, Weizmann Institute of Science, Rehovot, Israel. Segal chaired a session on this topic, and said he was disappointed not to see the reported MRI changes correlated with cognitive deficits, which are readily obtainable with mice. Such data would have strengthened the results, he told Alzforum.—Gwyneth Dickey Zakaib.
References
News Citations
- eFAD Research Surprise: In Mutation Carriers, Amyloid Starts in Striatum
- Animals for PET: Amyloid Imaging in Mice
Paper Citations
- Grueninger F, Bohrmann B, Czech C, Ballard TM, Frey JR, Weidensteiner C, von Kienlin M, Ozmen L. Phosphorylation of Tau at S422 is enhanced by Abeta in TauPS2APP triple transgenic mice. Neurobiol Dis. 2010 Feb;37(2):294-306. PubMed.
- Howlett DR, Richardson JC, Austin A, Parsons AA, Bate ST, Davies DC, Gonzalez MI. Cognitive correlates of Abeta deposition in male and female mice bearing amyloid precursor protein and presenilin-1 mutant transgenes. Brain Res. 2004 Aug 13;1017(1-2):130-6. PubMed.
- Acosta-Cabronero J, Alley S, Williams GB, Pengas G, Nestor PJ. Diffusion tensor metrics as biomarkers in Alzheimer's disease. PLoS One. 2012;7(11):e49072. PubMed.
Other Citations
Further Reading
Papers
- Grand'maison M, Zehntner SP, Ho MK, Hébert F, Wood A, Carbonell F, Zijdenbos AP, Hamel E, Bedell BJ. Early cortical thickness changes predict β-amyloid deposition in a mouse model of Alzheimer's disease. Neurobiol Dis. 2013 Jun;54:59-67. PubMed.
- Kim JH, Ha TL, Im GH, Yang J, Seo SW, Lee IS, Lee JH. Magnetic resonance imaging of amyloid plaques using hollow manganese oxide nanoparticles conjugated with antibody aβ1-40 in a transgenic mouse model. Neuroreport. 2013 Jan 9;24(1):16-21. PubMed.
- Kerbler GM, Hamlin AS, Pannek K, Kurniawan ND, Keller MD, Rose SE, Coulson EJ. Diffusion-weighted magnetic resonance imaging detection of basal forebrain cholinergic degeneration in a mouse model. Neuroimage. 2012 Nov 2;66C:133-141. PubMed.
- Wang FH, Appelkvist P, Klason T, Gissberg O, Bogstedt A, Eliason K, Martinsson S, Briem S, Andersson A, Visser SA, Ivarsson M, Lindberg M, Agerman K, Sandin J. Decreased axonal transport rates in the Tg2576 APP transgenic mouse: improvement with the gamma-secretase inhibitor MRK-560 as detected by manganese-enhanced MRI. Eur J Neurosci. 2012 Nov;36(9):3165-72. PubMed.
News
- Animals for PET: Amyloid Imaging in Mice
- Research Brief: If the Cap Fits...? Portable Scanners for Rodent PET
- Of Mice and Men—Functional Imaging of Aβ Toxicity, Early Pathology
- Individual Plaques Reveal Themselves in Mouse Brain MRI
- Visualizing Success with MRI of Amyloid Plaques in Live Mice
- Dog ADNI? Companies to Build Pre-Competitive Canine Model
- eFAD Research Surprise: In Mutation Carriers, Amyloid Starts in Striatum
- Tau, α-Synuclein Spread: Crazy Stuff—How Might It Work?
- Like Star Born of Supernova, Plaque Born of Exploded Neuron?
- Can Dousing PyroGlu-Aβ Treat Alzheimer’s Disease?
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.