Tau PET: The Field Expands Rapidly
Quick Links
As the field of Alzheimer’s and related dementias is trying to go all-in on detecting—and targeting—tau pathology, scientists are still grappling with exactly how to deploy PET imaging in the effort. A still-new, and still-limited set of tools, existing tau tracers vary quite a bit in terms of their sensitivity, off-target binding, and exactly which form of tau they recognize. The 14th Human Amyloid Imaging conference, held January 15–17 in Miami, brought together researchers for the latest characterization of these agents, as well as for some contrast-and-compare data sharing. The conference was organized by Keith Johnson of Massachusetts General Hospital, Boston, William Jagust of the University of California, Berkeley, William Klunk and Chester Mathis of the University of Pittsburgh, and Maria Carrillo of the Alzheimer’s Association.
- Tau tracers PI-2620 and APN-1607 are the first to strongly bind 4R tau.
- MK-6240 is highly sensitive, suggesting it can detect tangles early on.
- Human trials are starting for new tracers JNJ-067 and SNFT-1.
One upshot from a diverse array of presentations: PI-2620 and APN-1607 might recognize the four-repeat (4R) aggregates of tau that are found in non-AD tauopathies such as progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD). Another: Flortaucipir off-target binding may be a bit more extensive than previously thought. Physical activity during the tracer uptake period seemed to lower nonspecific binding, however, suggesting one way to manage this. Finally: Some researchers argued that MK-6240 is more sensitive than other tracers, and two new tracers—JNJ-067 and SNFT-1—made their entré. Read below for a summary.
Do We Have a First Tracer For 4R Tau?
Four current tracers—flortaucipir, MK-6240, RO-948, and GTP1—detect, with high specificity, the paired helical filaments of 3/4R tau that are present in the Alzheimer’s brain, but they bind weakly, if at all, to the 4R tau fibrils that mark PSP and CBD (Sep 2016 conference news; Dec 2016 conference news). Initially, scientists were disappointed that tau PET might not work in non-AD tauopathies, but appreciated starting out with tracers that distinguished between different types of tauopathy (Sep 2018 news). Now, they appear to be getting a tool for 4R tau, after all, in Life Molecular Imaging’s PI-2620. In Miami, Matthias Brendel of University Hospital Munich noted that in autoradiography studies, the tracer binds to the globus pallidus and putamen in sections from PSP brains, but not controls.
Brendel and colleagues scanned 60 clinically diagnosed PSP patients and 10 controls at three centers in Germany, one in Australia, and one in the United States. The former comprised 40 people with classic PSP symptoms, aka Richardson’s syndrome, and 20 with atypical symptoms. Four-R tau predominates more in Richardson’s syndrome than in atypical PSP (Williams et al., 2005), and PI-2620 retention reflected this. People with PSP-RS had elevated uptake in all brain regions affected by this disease: the globus pallidus, putamen, subthalamic nucleus, substantia nigra, and dentate nucleus. The atypical PSP group had elevated signal only in the globus pallidus and subthalamic nucleus, and their signal was less intense than in PSP-RS brains. Overall, a semiquantitative read of PI-2620 scans distinguished PSP-RS cases from controls with a sensitivity and specificity of 85 and 83 percent, respectively. For atypical PSP, sensitivity and specificity were 60 and 83 percent, respectively. The findings suggest this tracer could aid PSP diagnosis, Brendel suggested.
In a separate study of 10 controls and 32 people with corticobasal degeneration, patients again had elevated tracer signal in the globus pallidus, putamen, subthalamic nucleus, and dentate nucleus, with a trend for higher binding in the frontal cortex. Dentate nucleus binding in these patients tracked with how severe their symptoms were, and cortical binding with how long they had been symptomatic. For 23 of the CBD patients, their scans were positive by visual read alone, suggesting the tracer might be readily adapted for diagnosing this disorder, as well.

More Inclusive Tracer? PI-2620 binds to 4R aggregates in the tauopathies PSP (second row) and CBD (third row), and to paired helical filaments of tau in AD (bottom row), while having little background in healthy controls (top row). [Courtesy of Roesler et al., Progress in Neurobiology.]
While PI-2620 binds both 3/4R and 4R tau aggregates, it does so with different kinetics, Brendel reported in Miami. He compared tracer uptake in 14 amyloid-positive participants, 14 with suspected CBD, and 15 with suspected PSP. The latter two groups bound less tracer in cortex and cleared it faster than the amyloid-positive people. The data imply that PI-2620 binds with lower affinity to 4R than 3/4R tau. This difference could help distinguish these two types of tau pathology in imaging studies, Brendel believes. This is relevant, because patients with a clinical diagnosis of CBD can have either type of tau aggregate.
Lest it seem progress was easy or straightforward, some data presented in Miami cast doubt on PI-2620’s ability to detect 4R tau. Cinthya Agüero at Massachusetts General Hospital, Boston, compared binding of flortaucipir, MK-6240, and PI-2620 in the same set of postmortem samples. She reported that all three bound strongly to tangles in AD brain, but not in non-AD tauopathies such as Pick’s disease, progressive supranuclear palsy, corticobasal degeneration, and chronic traumatic encephalopathy. What gives? Brendel noted that the Boston group used cryopreserved tissue sections, while his group used paraffin embedding. In preliminary experiments on cryopreserved sections from PSP brains, Brendel, too, sees a weaker PI-2620 signal than in paraffin sections. Thus, this could be a methodological difference, he said.
Flortaucipir Off Target
At this stage in tau PET research, one of the bigger worries with detecting 3/4R tau, i.e. Alzheimer’s tangles, is off-target binding that may confound the interpretation of a person’s scan. Just how serious a problem is it? Flortaucipir, the only one of the first-generation tau tracers still standing, binds nonspecifically to basal ganglia and choroid plexus. Choroid-plexus binding is problematic because its signal could bleed into the hippocampus lying just below it, potentially inflating the estimation of AD pathology.
In Miami, Shaney Flores of Washington University in St. Louis reported that, in the hands of the WashU group, flortaucipir binds to the skull, as well. Among 216 amyloid-negative and 97 amyloid-positive participants, about 16 percent had high flortaucipir uptake in the skull. This binding was most pronounced in inferior occipital and temporal regions. It did not correlate with the intensity of other off-target binding, suggesting it recognizes a unique target. Inferior temporal regions are an early site of tau accumulation in AD. In the WashU cohort, most of those with skull uptake did not have amyloid pathology, suggesting the signal could potentially lead to a false-positive AD diagnosis.
In fact, off-target binding to the skull or meninges might explain some gender differences that were previously reported with flortaucipir scans, said Davneet Minhas of the University of Pittsburgh. The UPitt group examined flortaucipir scans from 162 people scanned there, plus 169 ADNI participants. They were cognitively healthy or had MCI. In both cohorts, women had higher uptake than men around the perimeter of the brain but not in the brain, possibly clouding comparisons of tau pathology between the sexes. Several studies had previously reported higher flortaucipir uptake in women (Feb 2019 news; Nov 2019 news).
Now for some good news: It appears off-target binding can be held at bay by physical activity in the time between a person’s injection and scan, i.e., while the tracer is traveling into the brain and finding its targets there. According to Hoon-Ki (Paul) Min of the Rochester Mayo Clinic in Minnesota, among a cohort of 330 people scanned with flortaucipir, those who reported napping during this 80-minute uptake period had higher binding to bone and meninges than those who remained active. The more sleep a person reported getting, the higher this off-target binding was. On the other hand, participants who walked around during this period had less uptake in these regions, as well as less off-target binding in basal ganglia, than those who stayed sedentary.
Scientists in Sweden flagged an additional type of off-target binding. Antoine Leuzy of Lund University, Malmö, directly compared flortaucipir and RO-948 uptake in the same three people with semantic variant primary progressive aphasia (svPPA), a form of FTD distinguished by TDP-43 pathology rather than tau tangles. Flortaucipir lit up the lower temporal lobe, whereas RO-948 did not. Which molecule in the temporal lobe might generate flortaucipir’s off-target signal is a mystery. A previous study found little evidence for flortaucipir binding TDP-43 (Smith et al., 2019). One possibility is that flortaucipir is binding to MAO-B, which is expressed by reactive astrocytes, Leuzy noted. “In common with most next-generation tau tracers, RO-948 seems to have a better off-target binding profile and broader dynamic range than flortaucipir,” he wrote to Alzforum (Smith et al., 2020).
Meanwhile, Milos Ikonomovic of UPitt led a postmortem study that correlated the flortaucipir PET signal with region-matched ELISA concentrations of total tau and tau phosphorylated at Ser396, Ser199, or Thr231. He did this in autopsy brains from three people who had had Alzheimer’s dementia and one cognitively normal person with AD pathology. Flortaucipir PET correlated with the ratio of pSer396 to total tau and pSer199 to total tau in the three people who had been impaired, but not in the one who had died while still cognitively normal. In one brain from a cognitively impaired person with severe cortical tangle pathology, flortaucipir PET also correlated with the ratio of pThr231 to total tau.

Broader Range. MK-6240 (top) generates a higher signal than flortaucipir (bottom) in the same AD patients. [Courtesy of the University of Pittsburgh PPG/ADRC.]
MK-6240, the Sensitive Guy Among the Lot?
With so many tau tracers being studied, which one is best? It may depend on what exactly you need it for. MK-6240 detects tau tangles present in AD brain with an affinity of 0.3 nM, which is considered high. Some HAI presentations suggested that MK-6240 is particularly sensitive to low levels of tangles, and thus might be suitable for early detection.
Brian Lopresti of UPitt directly compared flortaucipir and MK-6240 uptake in the same five people with AD, one with MCI, and nine controls. They evaluated six brain regions per person, for 90 total reads. In this study, MK-6240 had twice the dynamic range of flortaucipir in target regions, while background uptake in cerebellum was similar for both tracers. Even so, readers interpreted flortaucipir and MK-6240 scans similarly, making the same diagnostic call in 13 of the 15 people. Where the calls were discordant in specific brain regions, flortaucipir was more often read as positive than MK-6240, perhaps due to noise. The two tracers had distinct patterns of off-target binding, with flortaucipir lighting up striatum and choroid plexus, MK-6240 meninges. Both had some binding to bone.
How problematic is the meningeal binding? In Miami, Justin Sanchez of Mass. General reported that about half of clinically normal people have some extra-cerebral MK-6240 uptake that forms a halo around the brain in scans. This signal closely apposes the entorhinal cortex, a region of early tangle accumulation, with a proximity near the limit of spatial resolution. That said, this off-target signal can be distinguished from cortical uptake, Sanchez said. The trick? An analytic method that examines cortical surface projections, rather than volume-based regions of interest.
Sanchez further noted that MK-6240’s on-target cortical signal correlates with age and amyloid load, whereas its extra-cerebral signal does not. The two types of uptake follow different time courses; the on-target signal plateaus, while the extra-cerebral signal continues to rise over two hours. “We are optimistic about the ability of the surface projection method to distinguish tau-like from non-tau-like signals, and to optimize our sampling of cortical MK-6240 signal,” Sanchez wrote to Alzforum.
Firoza Lussier of McGill University in Verdun, Canada, examined MK-6240 uptake in 12 people with a familial AD mutation and in 11 noncarriers. Most of the carriers had a PS1 mutation; six of them were symptomatic. In carriers, higher tracer uptake correlated with how close a person was to his or her estimated year of onset (EYO). Regionally, this correlation was significant in the entorhinal cortex, right posterior cingulate, and precuneus. The MK-6240 signal could be detected as many as 10 years before EYO, hinting that this tracer might allow for early detection. In DIAN, which thus far has reported results with flortaucipir, the tau signal became apparent some years later, closer to a carrier’s EYO (Aug 2017 conference news).
Finally, Zhizhen Zeng of Merck reported that MK-6240 can detect tangles in Parkinson’s disease. In postmortem sections from 36 PD brains, binding of MK-6240 to the substantia nigra, caudate, and putamen matched immunostaining with antibody AT8, which recognizes paired helical filaments of tau. In all, 18 of 22 substantia nigra samples and nine of 13 caudate and putamen samples bound MK-6240. Off-target binding was limited to cells containing neuromelanin, Zeng reported. About half of Parkinson’s patients have notable tangle accumulation at autopsy, but flortaucipir shows little signal in these patients (Irwin and Hurtig, 2018; Smith et al., 2018; Hansen et al., 2020). “The results of this study show that there is significant tau pathology in the striatum and substantia nigra in almost all of the postmortem PD brains we sampled,” Eric Hostetler of Merck wrote to Alzforum. He noted that this has implications for development of an α-synuclein tracer, suggesting that that ligand will have to be selective for α-synuclein over tau tangles.
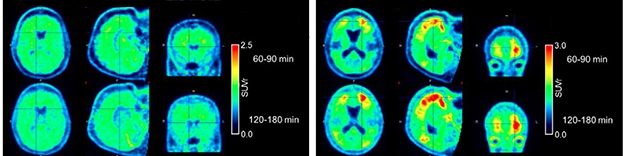
Low Background. The new tracer JNJ-067 distinguishes cleanly between control (left) and Alzheimer’s (right) brains. [Courtesy of Invicro, a Konica Minolta company.]
Meet the New Tau Tracers
Additional tau tracers are in development. In preclinical studies, Janssen’s JNJ-067 showed higher affinity than flortaucipir for tangles (Apr 2017 conference news), and in Miami, Janssen’s Mark Schmidt presented new data from scans of five amyloid-positive Alzheimer’s patients and five healthy age-matched controls. Four of the AD patients bound the tracer in their cortices. Controls had negative scans, except one person, who had focal hotspots in the occipital cortex around shotgun pellets lodged there from an old hunting accident.
JNJ-067 is in a Phase 1 trial conducted at the University of California, Berkeley, and Suzanne Baker of Lawrence Berkeley National Laboratory, California, reported preliminary findings at HAI. So far, four healthy controls, three people with AD, three with amyloid-positive MCI, and three with progressive supranuclear palsy have been scanned. Baker noted that JNJ-067 appears “cleaner” than flortaucipir, without off-target binding to choroid plexus and less affinity for MAO-B. Unlike other second-generation tau tracers, it does not appear to bind meninges. However, JNJ-067 did display off-target binding in midbrain, basal ganglia, and white matter, Baker reported.
In AD patients, JNJ-067 lit up the entorhinal cortex and widespread cortical regions, as expected. This on-target signal was higher in people whose MMSE scores were lower, but it was a generally weaker signal than seen with MK-6240 and PI-2620, Baker said. Schmidt agreed that JNJ-067 has a similar dynamic range to flortaucipir, smaller than that of MK-6240.
One MCI participant had focal cortical uptake of JNJ-067, but none had any signal in entorhinal cortex. Baker noted that one-third of ADNI MCI participants have no tracer uptake in this early cortical region, either, so it is too early to draw conclusions about the ability of this new tracer to detect MCI. So far, JNJ-067 has not labeled any brain areas in PSP patients. Most current tau tracers detect only 3/4R tau, because they were selected for binding to AD brain tissue.
In regions with high tauopathy burden, JNJ-067’s kinetics are slow, reaching steady state in about two hours. This is common with high-affinity tracers, Schmidt said, and is true for flortaucipir and MK-6240, as well. Janssen plans to make the tracer available via a royalty-free license to academic and industry groups, Schmidt said. At the moment, it is being produced at Invicro and Berkeley.
The first-generation tau tracer THK-5351, developed at Tohoku University in Sendai, Japan, was felled by nonspecific binding to the enzyme MAO-B (Apr 2017 conference news). Since then, researchers there have identified a new candidate, THK-5562, now renamed SNFT-1. In Miami, Ryuichi Harada of Tohoku reported that SNFT-1 selectively binds tau aggregates over other proteins, including Aβ, α-synuclein, TDP-43, MAO-A, and MAO-B. In autoradiography of postmortem human brain sections, SNFT-1 had a strong signal in cortex but not basal ganglia. The tracer quickly entered control mouse brain after intravenous injection, and washed out quickly, too. Researchers are now optimizing radiosynthesis for clinical study, Harada wrote to Alzforum.
Meanwhile, Aprinoia Therapeutics in Taipei, Taiwan, is developing a derivative of the discontinued tau tracer PBB3. Initially known as PM-PBB3 or MNI-958, the derivative is now called APN-1607 and is in Phase 1 in Taiwan. The compound is light-sensitive. This complicates its production and use, but this can be overcome with longer wavelength, i.e. red/yellow, lighting.
In Miami, Ing-Tsung Hsiao of Chang Gung University in Taoyuan, Taiwan, reported preliminary data comparing APN-1607 with THK-5351, not to its own predecessor PBB3. Unlike THK-5351, APN-1607 did not light up basal ganglia or thalamus in healthy controls or AD patients. The new tracer appeared to more faithfully reflect pathology than the older one, Hsiao showed. In 10 AD patients, APN-1607 uptake varied more than THK-5351 did between brain regions expected to have high and low tangle load, and it matched Braak staging. In five controls, meanwhile, APN-1607 showed but a low, uniform background signal, without the hot spots of off-target binding seen with THK-5351. The findings suggest APN-1607 would have more diagnostic power than THK-5351, Hsiao noted. APN-1607 does not bind MAO-A or MAO-B in competition assays. In a separate study of three people who had had a stroke, the new tracer, unlike THK-5351, did not bind to ischemic lesions.
Finally, APN-1607 may recognize 4R forms of tau. In Miami, Richard Margolin of Aprinoia reported that the tracer’s signal matched 4R histopathology in the tau mouse model rTg4510. So far, researchers have scanned 45 people with PSP and nine with CBD with APN-1607. Binding patterns matched the expected distribution of tangles and correlated with the clinical severity of PSP, Margolin said. Likewise, in 40 people with AD, APN-1607 uptake showed the expected pattern of binding and correlated with disease severity. Uptake in 75 healthy controls scanned so far has been low. APN-1607 does not bind meninges, but does bind to choroid plexus.—Madolyn Bowman Rogers
References
News Citations
- Fluid NfL Shines, Tau PET Dims, in the Hunt for FTD Biomarkers
- Tau PET in Down’s: Unique Patterns Among Alzheimer’s Types and Stages
- Flortaucipir Imaging Distinguishes Alzheimer’s From Other Disorders
- Is a Woman’s Brain More Susceptible to Tau Pathology?
- ApoE4 and Tau in Alzheimer’s: Worse Than We Thought? Especially in Women
- Data from DIAN Revise Familiar Biomarker Trajectories
- Next-Generation Tau PET Tracers Strut Their Stuff
Research Models Citations
Paper Citations
- Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, Holton JL, Revesz T, Lees AJ. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson's syndrome and PSP-parkinsonism. Brain. 2005 Jun;128(Pt 6):1247-58. Epub 2005 Mar 23 PubMed.
- Smith R, Santillo AF, Waldö ML, Strandberg O, Berron D, Vestberg S, van Westen D, van Swieten J, Honer M, Hansson O. 18F-Flortaucipir in TDP-43 associated frontotemporal dementia. Sci Rep. 2019 Apr 15;9(1):6082. PubMed.
- Smith R, Schöll M, Leuzy A, Jögi J, Ohlsson T, Strandberg O, Hansson O. Head-to-head comparison of tau positron emission tomography tracers [18F]flortaucipir and [18F]RO948. Eur J Nucl Med Mol Imaging. 2020 Feb;47(2):342-354. Epub 2019 Oct 14 PubMed.
- Irwin DJ, Hurtig HI. The Contribution of Tau, Amyloid-Beta and Alpha-Synuclein Pathology to Dementia in Lewy Body Disorders. J Alzheimers Dis Parkinsonism. 2018;8(4) Epub 2018 Aug 10 PubMed.
- Smith R, Schöll M, Londos E, Ohlsson T, Hansson O. 18 F-AV-1451 in Parkinson's Disease with and without dementia and in Dementia with Lewy Bodies. Sci Rep. 2018 Mar 16;8(1):4717. PubMed.
- Hansen AK, Parbo P, Ismail R, Østergaard K, Brooks DJ, Borghammer P. Tau Tangles in Parkinson's Disease: A 2-Year Follow-Up Flortaucipir PET Study. J Parkinsons Dis. 2020;10(1):161-171. PubMed.
External Citations
Further Reading
News
- PET Identifies Tangles Decades after a Single Traumatic Brain Injury
- Autopsy Study Confirms Flortaucipir PET Lights Up Tau Pathology
- It’s Official: Tau PET Sees Tangles, and Staging Tangles Predicts Decline
- Flortaucipir Imaging Distinguishes Alzheimer’s From Other Disorders
- Flortaucipir Meets Primary Endpoints in Phase 3 Autopsy Trial
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.