Rare Luck: Two Copies of ApoE2 Shield Against Alzheimer’s
Quick Links
Mention ApoE and Alzheimer’s, and the conversation turns to the E4 allele, the strongest susceptibility gene for the disease. But ApoE has another side, in ApoE2. Though this isoform protects against AD, scientists have barely studied it. Now ApoE2 is attracting scrutiny as scientists are asking exactly how some people maintain their mental acuity into old age. At the Alzheimer’s Association International Conference, held July 14–18 in Los Angeles, a study of ApoE genotypes in 5,000 autopsy-confirmed cases of AD revealed that people with two copies of E2 see their risk of dementia plummet by a stunning 90 percent compared with those with the common E3/E3 genotype. Other work suggested that this could be because ApoE2 reduces amyloid and tau pathology, and boosts gray-matter volume in critical brain regions. E2’s benefits seem specific to Alzheimer’s, not generic to neurodegeneration.
ApoE is the major cholesterol-carrying protein in the brain. It has been studied since its discovery as an AD risk gene in the early 1990s, but is newly emerging as a hub for glial responses to amyloid and tau aggregate deposition (Aug 2018 news; Sept 2017 news). The gene exists as three polymorphic alleles—E2, E3, and E4—with a worldwide frequency of 8 percent, 78 percent, and 14 percent, respectively. Several mutated forms are also known, for example the so-called Heidelberg, Pittsburgh, and Christchurch mutations (Feussner et al., 1992; Kamboh et al., 1999; Wardell et al., 1987).
ApoE4 receives by far the most attention from AD researchers, because it boosts the risk of AD up to 15-fold depending on the study population, and occurs in 40 percent of people with AD. E2, the protective allele, has received scant attention, because it is the least common of the three and largely absent from AD samples. People with one copy of E2 have half the chance of developing AD compared with those with the more common E3/E3 genotype. But does the additional E2 have an effect beyond that? “We have not known whether E2 dose has a differential risk, i.e. whether E2/2 risk is significantly lower than E2/3,” Eric Reiman said in presenting the study.
To find out, Reiman and colleagues at Banner Alzheimer’s Institute, Phoenix, Gyungah Jun at Boston University, Joseph Arboleda of Massachusetts Eye and Ear, Yakeel Quiroz of Massachusetts General Hospital, and colleagues from the AD Genetics Consortium decided to look at data from a lot of brains. They analyzed the contribution of all three ApoE alleles to dementia risk and pathology in 5,007 brains from the ADGC. This sample included 4,018 autopsy-confirmed Alzheimer’s dementia cases, plus 989 pathologically and cognitively unaffected donors. Besides providing sufficient numbers to study E2/E2 homozygotes, this cohort avoids the confounding issue of misdiagnosis by eliminating dementias not due to AD, as well as people who had AD pathology at the time of death but no dementia.
As expected, ApoE2 homozygotes were rare—numbering just 24 out of more than 5,000 people, or 0.5 percent. Compared with other genotypes, they were far less likely to have AD. E2 homozygotes made up 0.1 percent of cases but 1.9 percent of healthy controls. In contrast, ApoE4/4 homozygotes accounted for 15.6 percent of cases and only 1 percent of controls. In other words, 19 of the 24 ApoE2/2s were cognitively healthy, but only 10 of 633 ApoE4/4s were.
E2 homozygotes had a 66 percent risk reduction compared even with E2/3 carriers, an 87 percent risk reduction compared to the most common genotype, E3/3, and a whopping 99.6 percent risk reduction compared to people who were E4/4. Basically, most people with E4/4 get Alzheimer’s dementia, while few with ApoE 2/2 do.
Having two E2 alleles correlated with less amyloid plaque and tau neurofibrillary tangle pathology. ApoE2’s protective effect on tau pathology was still apparent even when adjusted for amyloid plaque load. That mirrors recent animal data suggesting that ApoE4 acts to worsen tau pathology independent of amyloid (Shi et al., 2017).
The new study’s main finding echoes that of a previous study by Pieter-Jelle Visser, Maastricht University, the Netherlands, and colleagues. They had found 16 ApoE2/2 carriers in a sample of 7,583 people, of whom 10 were cognitively normal and amyloid-negative, whereas 301 of the 386 ApoE4/4 carriers in the sample had Alzheimer’s dementia. This sample, however, was not pathology-confirmed (Jansen et al., 2015).
How important is neuropathology confirmation? Reiman et al. compared the risk estimates derived from the autopsy-verified cohort to those calculated from a cohort of 23,857 living people who were clinically diagnosed as having probable AD dementia or being cognitively normal, and were of unknown amyloid status. In that analysis, the protective effect of 2/2, and the increased risk due to 4/4, both were underestimated. For example, the scientists found that the odds ratio associated with E4/E4 over E3/E3 in the clinical sample was 10.7, versus 31.22 in the pathologically confirmed cases. The dose effects for both E2 and E4 were also underestimated in the living sample. Thus, the autopsy analysis provides updated, and likely more accurate, risk estimates for all ApoE genotypes, Reiman and colleagues believe, at least for these non-Hispanic white research participants.
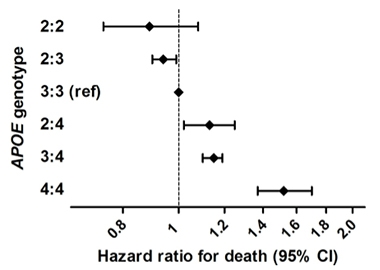
Life Extending. In a large epidemiological study combining six population-based cohorts of people with European ancestry, ApoE2 homozygotes were at lower risk of dying than other genotypes. ApoE4 homozygotes were at greatest risk. [Courtesy of Wolters et al., PLoS One 2019.]
ApoE’s impact on Alzheimer’s is known to differ among ethnic groups. This type of study is needed with more diverse subjects, more E2/2 homozygotes, and taking into account possible effects of the ApoE alleles on survival, which could skew the results. Nonetheless, Reiman said, “Our results suggest that ApoE2 homozygosity is associated with an exceptionally low risk of AD, that the impact of APOE and its variants on AD risk is significantly greater than previously appreciated, and that there is a compelling reason to discover treatments that promote this protective effect.”
That jibes with recent results from Sudha Seshadri, University of Texas Health, San Antonio, Frank Wolters, Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues. They found a survival advantage for ApoE2 carriers in a study of 38,537 people from six population-based cohorts (Wolters et al., 2019). They identified 239 E2 homozygotes, who led the longest lives. The effect of E2 was only partly explained by its effects on blood lipids or vascular disease. E4 homozygotes had the highest risk of death, and this was largely accounted for by its association with dementia. Seshadri told Alzforum that the investigators will look at AD and cognitive change, and also at amyloid and tau PET measures in this sample.
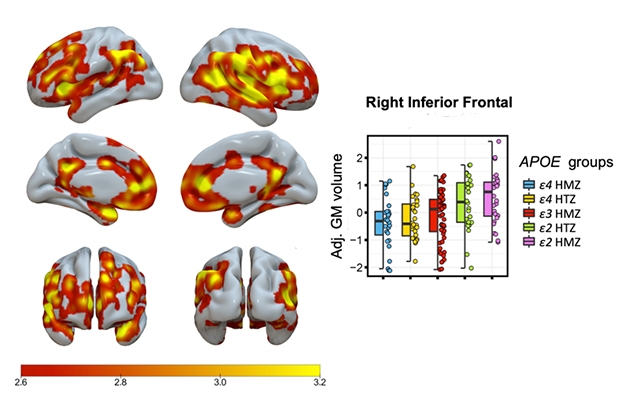
Bit by Bit. Shading indicates areas with a significant stepwise, ApoE genotype-related increase in gray-matter volume, with E4/4 homozygotes having the lowest and E2/2s the highest volume. Graphic shows data for one brain region. [Image courtesy of Gemma Salvadó.]
How might ApoE2 bestow resilience? To find out, Gemma Salvadó, Barcelonaβeta Brain Research Center, Spain, presented a study where she and co-authors gathered together imaging data from different samples on as many older ApoE2 homozygotes as they could. Their goal was to compare the brain structure of E2/2s with that of other ApoE genotypes.
Previously, E2 had been linked to subtle changes in brain morphology in healthy people, including slower hippocampal atrophy in old age, and larger hippocampi in middle age (Chiang et al., 2010; Fennema-Notestine et al., 2011). In childhood, E2 carriers have been reported to have thicker entorhinal cortices than E3 homozygotes or E4 carriers (Shaw et al., 2007). But these studies all focused on people with one copy of E2.
To find out what that extra E2 would do, Salvadó collected and analyzed MRI data on cognitively unimpaired people in the ALFA study in Barcelona (Molinuevo et al. 2016), the Amsterdam University Medical Center cohort, OASIS open-access imaging studies, and ADNI. She found 28 E2/E2 homozygotes. She matched each of them with five other subjects from the same center on age, sex, and education level, and one of every other ApoE genotype. That gave a total of 168 subjects, with a mean age of 62.
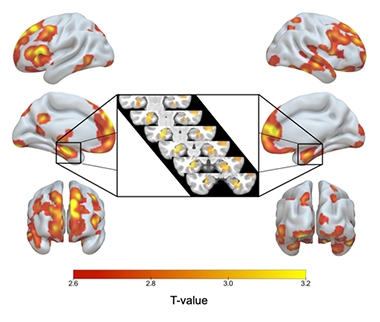
ApoE2/2 Over 3/3. Red to yellow shading indicates brain regions where ApoE2 homozygotes had more gray matter than E3 homozygotes, including both hippocampi (inset). [Image courtesy of Gemma Salvadó.]
Compared with their E3/3 matches, the E2/2s had larger gray-matter volume in their hippocampi and other AD signature areas, including the medial temporal cortex, inferior temporal, temporal pole, precuneus, and superior parietal regions. E2 homozygotes also had more gray matter in areas related to cognitive resilience in aging, namely in the anterior cingulate and medial prefrontal areas (Arenaza-Urquijo et al., 2019; Harrison et al., 2018). When compared with E2/3 heterozygotes, the E2 homozygotes boasted few significant differences in gray matter, but they did have even larger hippocampi than the 2/3s.
Salvadó reported a stepwise, genotype-related increase in gray-matter volume, with E4/4 homozygotes having the lowest, E3/3s having intermediate, E2/3s higher, and E2/2s the highest volume (see image below).

ApoE2/2 Over 2/3. Compared with the next-protective genotype, ApoE2/3, E2 homozygotes have more gray matter only in a few small areas.
She believes the larger gray matter in strategic brain areas may help E2 homozygotes cope with AD pathology, if and when it appears. Because ApoE2 plumps up the entorhinal cortex already in childhood, resilience may spring from developmental processes, she said.
“These are very important findings,” Reiman commented. “They suggest that if atrophy is like eroding the tread in tires, E2 homozygotes may start with more tread,” he said. Salvadó is trying to expand the study to look at more homozygotes from additional cohorts, and other imaging modes.
Terry Goldberg, Columbia University, New York, has been studying potential mechanisms of ApoE2-mediated neuroprotection for years (Conejero-Goldberg et al., 2014). At AAIC, Goldberg presented results on the relationship of ApoE alleles with neuropathology in AD, and extended the analysis to other diseases.
He used data on 1,557 brains from the National Alzheimer’s Coordinating Center database that had both clinical and neuropathological assessments. Because he had so few E2 homozygotes, Goldberg grouped E2/E2 and E2/E3 genotypes together, for a sample of 130. In that combined group, one in four brains had AD neuropathic changes, compared with 40 percent of E3/3s, 65 percent of E3/4s, and 85 percent of E4/4s. Consistent with previous work, E2 carriers had the mildest amyloid pathology, fewest neuritic plaques, and mildest tau pathology. A statistical mediation analysis suggested ApoE2’s association with reduced tangles went partly through its effect on amyloid, and was partly independent of amyloid. That dovetails with Reiman’s results.
In this dataset, too, ApoE2 strongly protected, even if Goldberg did not parse homozygotes: E2 cut the risk of amyloid and tau pathology by half compared with E3, and by 90 percent compared to E4.
Which is more powerful, E2 or E4? Comparing E2/E4 and E3/E4 genotypes, Goldberg found comparable levels of pathology. If anything, the E2/E4 pairing was worse. This suggests that E4 overwhelms the protective effects of E2. “You could say E4 is toxic,” Goldberg concluded. That suggests therapies using viral constructs to introduce E2 into E4-expressing brain tissue may not be helpful, he said.
What about other neurodegenerative diseases?
The literature offers mixed results on E2 and E4 in frontotemporal dementia. Some studies suggest E2 promotes risk (Mar 2016 news) while others credit it with protection and blame risk on E4 (Mishra et al., 2017). At AAIC, Goldberg reported that E2 was associated with more severe TDP-43 pathology among 103 cases of frontotemporal dementia, and with more tau pathology in 28 cases of Pick’s disease and 51 cases of progressive supranuclear palsy. He cautioned that these numbers are so small, the results could be spurious. Goldberg doesn’t know if these patients had C9ORF72 or progranulin mutations, and TDP-43 accumulation may be age-associated. In cases of α-synuclein pathology, Goldberg found that ApoE4 promotes, and E2 reduces, the spread of Lewy body pathology outside of its origin in the midbrain, into limbic and neocortical areas
Together, these studies highlight a renewed appreciation of the enormous impact ApoE exerts on the pathogenesis of AD (for example, see Wu and Zhao, 2016). The profound protection afforded by E2 will likely rekindle interest among drug developers, who have tried before and failed, but may now see fit to revisit this target.—Pat McCaffrey
References
News Citations
- ApoE: Common Microglial Culprit in Aging, Alzheimer’s, and Tauopathy?
- ApoE4 Makes All Things Tau Worse, From Beginning to End
- Et Tu, ApoE2? Paper Claims Allele Boosts Risk for Dementia—in ALS
Paper Citations
- Feussner G, Funke H, Weng W, Assmann G, Lackner KJ, Ziegler R. Severe type III hyperlipoproteinemia associated with unusual apolipoprotein E1 phenotype and epsilon 1/'null' genotype. Eur J Clin Invest. 1992 Sep;22(9):599-608. PubMed.
- Kamboh MI, Aston CE, Perez-Tur J, Kokmen E, Ferrell RE, Hardy J, DeKosky ST. A novel mutation in the apolipoprotein E gene (APOE*4 Pittsburgh) is associated with the risk of late-onset Alzheimer's disease. Neurosci Lett. 1999 Mar 26;263(2-3):129-32. PubMed.
- Wardell MR, Brennan SO, Janus ED, Fraser R, Carrell RW. Apolipoprotein E2-Christchurch (136 Arg----Ser). New variant of human apolipoprotein E in a patient with type III hyperlipoproteinemia. J Clin Invest. 1987 Aug;80(2):483-90. PubMed.
- Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg LT, Rojas JC, Gallardo G, Wang K, Roh J, Robinson G, Finn MB, Jiang H, Sullivan PM, Baufeld C, Wood MW, Sutphen C, McCue L, Xiong C, Del-Aguila JL, Morris JC, Cruchaga C, Alzheimer’s Disease Neuroimaging Initiative, Fagan AM, Miller BL, Boxer AL, Seeley WW, Butovsky O, Barres BA, Paul SM, Holtzman DM. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017 Sep 28;549(7673):523-527. Epub 2017 Sep 20 PubMed.
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ, Amyloid Biomarker Study Group, Aalten P, Aarsland D, Alcolea D, Alexander M, Almdahl IS, Arnold SE, Baldeiras I, Barthel H, van Berckel BN, Bibeau K, Blennow K, Brooks DJ, van Buchem MA, Camus V, Cavedo E, Chen K, Chetelat G, Cohen AD, Drzezga A, Engelborghs S, Fagan AM, Fladby T, Fleisher AS, van der Flier WM, Ford L, Förster S, Fortea J, Foskett N, Frederiksen KS, Freund-Levi Y, Frisoni GB, Froelich L, Gabryelewicz T, Gill KD, Gkatzima O, Gómez-Tortosa E, Gordon MF, Grimmer T, Hampel H, Hausner L, Hellwig S, Herukka SK, Hildebrandt H, Ishihara L, Ivanoiu A, Jagust WJ, Johannsen P, Kandimalla R, Kapaki E, Klimkowicz-Mrowiec A, Klunk WE, Köhler S, Koglin N, Kornhuber J, Kramberger MG, Van Laere K, Landau SM, Lee DY, de Leon M, Lisetti V, Lleó A, Madsen K, Maier W, Marcusson J, Mattsson N, de Mendonça A, Meulenbroek O, Meyer PT, Mintun MA, Mok V, Molinuevo JL, Møllergård HM, Morris JC, Mroczko B, Van der Mussele S, Na DL, Newberg A, Nordberg A, Nordlund A, Novak GP, Paraskevas GP, Parnetti L, Perera G, Peters O, Popp J, Prabhakar S, Rabinovici GD, Ramakers IH, Rami L, Resende de Oliveira C, Rinne JO, Rodrigue KM, Rodríguez-Rodríguez E, Roe CM, Rot U, Rowe CC, Rüther E, Sabri O, Sanchez-Juan P, Santana I, Sarazin M, Schröder J, Schütte C, Seo SW, Soetewey F, Soininen H, Spiru L, Struyfs H, Teunissen CE, Tsolaki M, Vandenberghe R, Verbeek MM, Villemagne VL, Vos SJ, van Waalwijk van Doorn LJ, Waldemar G, Wallin A, Wallin ÅK, Wiltfang J, Wolk DA, Zboch M, Zetterberg H. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015 May 19;313(19):1924-38. PubMed.
- Wolters FJ, Yang Q, Biggs ML, Jakobsdottir J, Li S, Evans DS, Bis JC, Harris TB, Vasan RS, Zilhao NR, Ghanbari M, Ikram MA, Launer L, Psaty BM, Tranah GJ, Kulminski AM, Gudnason V, Seshadri S, E2-CHARGE investigators. The impact of APOE genotype on survival: Results of 38,537 participants from six population-based cohorts (E2-CHARGE). PLoS One. 2019;14(7):e0219668. Epub 2019 Jul 29 PubMed.
- Chiang GC, Insel PS, Tosun D, Schuff N, Truran-Sacrey D, Raptentsetsang ST, Jack CR, Aisen PS, Petersen RC, Weiner MW, . Hippocampal atrophy rates and CSF biomarkers in elderly APOE2 normal subjects. Neurology. 2010 Nov 30;75(22):1976-81. PubMed.
- Fennema-Notestine C, Panizzon MS, Thompson WR, Chen CH, Eyler LT, Fischl B, Franz CE, Grant MD, Jak AJ, Jernigan TL, Lyons MJ, Neale MC, Seidman LJ, Tsuang MT, Xian H, Dale AM, Kremen WS. Presence of ApoE ε4 allele associated with thinner frontal cortex in middle age. J Alzheimers Dis. 2011;26 Suppl 3:49-60. PubMed.
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007 Jun;6(6):494-500. PubMed.
- Molinuevo JL, Gramunt N, Gispert JD, Fauria K, Esteller M, Minguillon C, Sánchez-Benavides G, Huesa G, Morán S, Dal-Ré R, Camí J. The ALFA project: A research platform to identify early pathophysiological features of Alzheimer's disease. Alzheimers Dement (N Y). 2016 Jun;2(2):82-92. Epub 2016 Mar 3 PubMed.
- Arenaza-Urquijo EM, Przybelski SA, Lesnick TL, Graff-Radford J, Machulda MM, Knopman DS, Schwarz CG, Lowe VJ, Mielke MM, Petersen RC, Jack CR, Vemuri P. The metabolic brain signature of cognitive resilience in the 80+: beyond Alzheimer pathologies. Brain. 2019 Apr 1;142(4):1134-1147. PubMed.
- Harrison TM, Maass A, Baker SL, Jagust WJ. Brain morphology, cognition, and β-amyloid in older adults with superior memory performance. Neurobiol Aging. 2018 Jul;67:162-170. Epub 2018 Mar 27 PubMed.
- Conejero-Goldberg C, Gomar JJ, Bobes-Bascaran T, Hyde TM, Kleinman JE, Herman MM, Chen S, Davies P, Goldberg TE. APOE2 enhances neuroprotection against Alzheimer's disease through multiple molecular mechanisms. Mol Psychiatry. 2014 Feb 4; PubMed.
- Mishra A, Ferrari R, Heutink P, Hardy J, Pijnenburg Y, Posthuma D, International FTD-Genomics Consortium. Gene-based association studies report genetic links for clinical subtypes of frontotemporal dementia. Brain. 2017 Apr 5; PubMed.
- Wu L, Zhao L. ApoE2 and Alzheimer's disease: time to take a closer look. Neural Regen Res. 2016 Mar;11(3):412-3. PubMed.
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.