Forget Fibrils: Lewy Pathology Is More Lipid Than Protein
Quick Links
In Parkinson’s disease, abnormal deposits called Lewy bodies and Lewy neurites pop up in neurons and processes, respectively. In 1998, Maria Grazia Spillantini at the University of Cambridge identified α-synuclein as their major component, providing a key clue to Parkinson’s pathogenesis (Spillantini et al., 1998). Yet despite decades of study, researchers still do not fully understand how these Lewy structures form and what they mean for the health of the cells that carry them. At the 14th International Conference on Alzheimer’s and Parkinson’s Diseases, held March 27–31 in Lisbon, Portugal, two talks from a European research group offered a surprising glimpse at the ultra-substructure of Lewy bodies.
- Lewy bodies and neurites are stuffed full of membranes, not protein filaments.
- α-Synuclein is phosphorylated in their periphery, truncated in the interior.
- Lewy bodies may form when excess α-synuclein disrupts membranes.
Using cutting-edge microscopy techniques, the researchers found that the center of most Lewy bodies appears to be stuffed with much more lipid than protein. In fact, they say, the deposits represent a mass of undigested membrane fragments, damaged organelles, and other cellular garbage. The researchers also shed new light on what forms of α-synuclein populate Lewy bodies, and how these forms are arranged within neurons. Together, the data offer new clues for deciphering the effect of these structures, as well as preventing formation of Lewy bodies, the speakers suggested. Both studies are currently in preprint form on bioRχiv.
“The two studies complement each other, and paint a sharper picture of Lewy pathology,” co-senior author Markus Britschgi of Roche in Basel, Switzerland, wrote to Alzforum.
Other researchers agreed the data advance the understanding of Lewy pathology. “These findings are exciting and should make us rethink what we have accepted for the past 30 years,” Tiago Outeiro of Göttingen University Medical Center, Germany, wrote to Alzforum (full comment below). Tim Bartels of the U.K. Dementia Research Institute at University College London noted that previous research had linked α-synuclein’s aberrant lipid interactions to cellular toxicity, but not to pathology. “By demonstrating that lipid dysfunction, but not necessarily amyloid formation, is the actual hallmark of the disease, these studies might reconcile past findings and refocus our research,” Bartels wrote (full comment below).
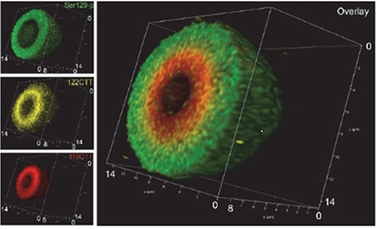
Layered Like an Onion? Antibodies to different forms of α -synuclein reveal concentric spheres of S129-phosphorylated (green), 122-C-terminally-truncated (gold), and 119-C-terminally truncated (red) α-synuclein inside a Lewy body. [Courtesy of Tim Moors.]
Previous research suggested that Lewy bodies form the way amyloid plaques do, with small oligomeric forms of α-synuclein coalescing to form fibrils. According to this theory, these fibrils entrap other proteins and eventually become compacted into a primarily filamentous deposit, the whole process taking up to 10 years. Older microscopic analysis of these bodies revealed a dense, protein-packed core and a peripheral halo of radiating filaments (Arima et al., 1998; Goedert, 2015). In addition to α-synuclein, Lewy bodies contain at least 90 other molecules, including mitochondrial proteins and autophagy-related proteins such as ubiquitin (Wakabayashi et al., 2012). Lewy bodies vary greatly in size, shape, and number between brain regions and donors, however, making them difficult to study.
New microscopic techniques now refine this picture. Academic researchers in Switzerland, the Netherlands, and Germany collaborated with Roche scientists to examine Lewy bodies from postmortem human brain samples. In Lisbon, Amanda Lewis of the University of Basel explained how the group correlated and overlaid scans from light and electron microscopy (EM) in the same block of tissue. This approach, established by Sarah Shahmoradian and led by Henning Stahlberg of the University of Basel, Wilma van de Berg of Amsterdam UMC, and Matthias Lauer and Britschgi at Roche, allowed them to make an unbiased EM survey of all Lewy bodies found by light microscopy. The researchers examined samples taken from the substantia nigra and CA2 of five PD patients. They found 17 Lewy bodies and three Lewy neurites in these samples.
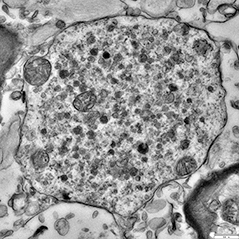
Lewy Flotsam. Electron microscopy unmasks a jumble of lipids, lysosomes (small dark circles), and damaged mitochondria (large circles) at the heart of most Lewy bodies. [Courtesy of Sarah Shahmoradian.]
Contrary to previous research, most of these Lewy bodies appear crammed with lipid structures, rather than proteinaceous filaments. Membrane fragments, vesicles, lysosomes, and misshapen organelles packed together in their cores. Mitochondria appeared frequently, and in some cases formed a shell around the inclusion. Scattered throughout the body were granular proteins, α-synuclein, and patches of disorganized filaments. The α-synuclein was mixed in with membranes and organelles, and did not appear to be in large aggregates.
Lewy neurites had a similar lipid-rich composition. In collaboration with Klaus Gerwert’s group at Ruhr University in Bochum, Germany, the researchers confirmed the presence of lipids using coherent anti-Stokes Raman scattering (CARS), an imaging technique that identifies the chemical composition of substances. Mass spectrometry and Fourier transform infrared (FTIR) spectroscopic imaging provided additional support for the finding.
Only three of the 17 Lewy bodies were stuffed with filaments. Of these, only one had the classic structure, with a protein-packed core surrounded by a filamentous net that traps organelles (Forno and Norville, 1976; Forno, 1996). The scientists are unsure if these were cytoskeletal or α-synuclein filaments (Shahmoradian et al., 2019). The findings raise the question of why previous EM studies had identified mostly filamentous Lewy bodies. It could be because lipid-filled bodies are difficult to distinguish in EM sections; thus, absent direct correlation of scans with light microscope images in this new study, these structures could have been missed. In addition, previous EM tissue preparation techniques tended to be harsh and strip lipids from samples, Britschgi told Alzforum.
The data supports a new theory for Lewy body formation, Lewis said. Perhaps excess α-synuclein disrupts membranes and impairs organelle trafficking, leading these types of debris to pile up. The mess could eventually compact into a Lewy body or Lewy neurite.
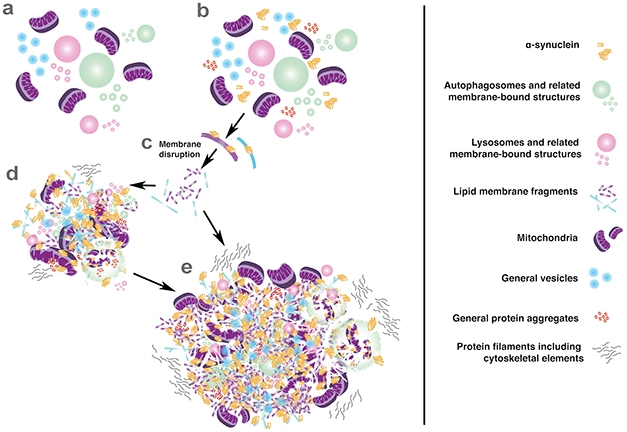
Trash Pile. New model of Lewy body formation suggests that α-synuclein wreaks havoc by disrupting membranes, then binds together a loose assortment of cellular garbage. [Courtesy of Sarah Shahmoradian.]
AD/PD attendees were intrigued. Some expressed skepticism, wondering if the data could be an artifact of tissue processing. Lewis said that in vitro controls show their processing methods do not alter tissue composition. In their paper, the authors demonstrate that cytoskeletal filaments and lipid-rich myelin sheaths are unharmed by the same tissue processing,
In the other talk, Tim Moors of Amsterdam UMC added detail on the role of α-synuclein in Lewy body inclusions. Led by van de Berg, Moors’ study combined immunostaining for specific forms of α-synuclein with stimulated emission depletion (STED) microscopy. STED produces an ultra-high resolution image by quenching surrounding fluorophores, leaving the region of interest highlighted. The researchers used antibodies against C-terminally truncated and Ser129 phosphorylated α-synuclein. Both forms are associated with Lewy bodies. Examining postmortem samples from the substantia nigra, hippocampi, and entorhinal cortices of 13 PD patients and six age-matched controls, they discovered a consistent pattern in spatial organization of α-synuclein in midbrain Lewy bodies.
The researchers found that most midbrain Lewy bodies had a layered, onion-like structure. The periphery typically contained pS129 α-synuclein caught in a cage of neurofilament. The dense core was stuffed with aggregated lipids and proteins, including truncated and membrane-associated α-synuclein. Lewy neurites had a similar structure. In the hippocampus and entorhinal cortex, on the other hand, α-synuclein staining appeared uniform throughout most Lewy bodies.

Neurofilament Wheel. A cage-like structure of neurofilament (yellow) encapsulates Lewy bodies. [Courtesy of Tim Moors.]
Do these forms of α-synuclein appear in healthy control brain? Barely any pS129 does, in keeping with this being a purely pathological form of the protein. However, controls did have some 122-truncated α-synuclein that associated with mitochondria, hinting this form could fulfill a physiological function (Moors et al., 2018).
To Moors’ mind, these data suggest that pS129 helps drive Lewy body formation. The researchers proposed that this starts with an excess of α-synuclein in the cytoplasm, which begins to clump with other proteins, membranes, and organelles. The cell may then phosphorylate α-synuclein to prevent fibril formation and trigger degradation of the garbage (Paleologou et al., 2008; Oueslati et al., 2013; Dahmene et al., 2017). When the mass becomes too indigestible, however, the cell encapsulates and compacts it using neurofilament. “α-Synuclein seems to follow its proposed physiological function of binding and organizing membranes, organelles, and vesicles in an orchestrated manner, yet under pathological conditions this seems to go havoc,” Britschgi suggested.
Though the studies focus on different aspects of Lewy bodies, they arrive at similar findings. “The unifying aspect of our two studies is the presence of organellar structures amid enriched different forms of α-synuclein,” Britschgi wrote to Alzforum. He added that the heterogeneity of Lewy pathology necessitates additional research with those new imaging methods. “We hope our findings trigger more studies on Lewy pathology, including mechanistic ones that will help us to identify therapies and biomarkers for Parkinson’s disease and related disorders,” Britschgi wrote.—Madolyn Bowman Rogers
References
Paper Citations
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998 May 26;95(11):6469-73. PubMed.
- Arima K, Uéda K, Sunohara N, Hirai S, Izumiyama Y, Tonozuka-Uehara H, Kawai M. Immunoelectron-microscopic demonstration of NACP/alpha-synuclein-epitopes on the filamentous component of Lewy bodies in Parkinson's disease and in dementia with Lewy bodies. Brain Res. 1998 Oct 12;808(1):93-100. PubMed.
- Goedert M. NEURODEGENERATION. Alzheimer's and Parkinson's diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015 Aug 7;349(6248):1255555. PubMed.
- Wakabayashi K, Tanji K, Odagiri S, Miki Y, Mori F, Takahashi H. The Lewy Body in Parkinson's Disease and Related Neurodegenerative Disorders. Mol Neurobiol. 2012 May 24; PubMed.
- Forno LS, Norville RL. Ultrastructure of Lewy bodies in the stellate ganglion. Acta Neuropathol. 1976 Mar 30;34(3):183-97. PubMed.
- Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996 Mar;55(3):259-72. PubMed.
- Shahmoradian SH, Lewis AJ, Genoud C, Hench J, Moors TE, Navarro PP, Castaño-Díez D, Schweighauser G, Graff-Meyer A, Goldie KN, Sütterlin R, Huisman E, Ingrassia A, Gier Y, Rozemuller AJ, Wang J, Paepe A, Erny J, Staempfli A, Hoernschemeyer J, Großerüschkamp F, Niedieker D, El-Mashtoly SF, Quadri M, Van IJcken WF, Bonifati V, Gerwert K, Bohrmann B, Frank S, Britschgi M, Stahlberg H, Van de Berg WD, Lauer ME. Lewy pathology in Parkinson's disease consists of crowded organelles and lipid membranes. Nat Neurosci. 2019 Jul;22(7):1099-1109. Epub 2019 Jun 24 PubMed.
- Moors TE, Maat CA, Niedieker D, Mona D, Petersen D, Timmermans-Huisman E, Kole J, El-Mashtoly SF, Zago W, Barbour R, Mundigl O, Kaluza K, Huber S, Hug MN, Kremer T, Ritter M, Dziadek S, Geurts JJ, Gerwert K, Britschgi M, van de Berg WD. Detailed structural orchestration of Lewy pathology in Parkinson’s disease as revealed by 3D multicolor STED microscopy. bioRχiv, November 14, 2018. bioRxiv.
- Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, Lansbury PT, Fernandez CO, Eliezer D, Zweckstetter M, Lashuel HA. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem. 2008 Jun 13;283(24):16895-905. PubMed.
- Oueslati A, Schneider BL, Aebischer P, Lashuel HA. Polo-like kinase 2 regulates selective autophagic α-synuclein clearance and suppresses its toxicity in vivo. Proc Natl Acad Sci U S A. 2013 Oct 8;110(41):E3945-54. PubMed.
- Dahmene M, Bérard M, Oueslati A. Dissecting the Molecular Pathway Involved in PLK2 Kinase-mediated α-Synuclein-selective Autophagic Degradation. J Biol Chem. 2017 Mar 3;292(9):3919-3928. Epub 2017 Jan 30 PubMed.
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
University Medical Center Goettingen
Our understanding of Lewy pathology has not changed much since 1912, when Fritz Heinrich Lewy first reported the typical types of accumulations in the brains of patients. Since 1997–98, we have focused primarily on the fact that α-synuclein appears to be one of the major components, but we have essentially ignored all the other proteins and components we know accumulate in Lewy bodies.
We were limited by the tools available, but also by the fact that any biochemical extraction is merely a simplification of what the real material really is. Therefore, new technologies, such as STED and other super-resolution techniques such as the ones several groups, including my own, are using, represent tremendous opportunities to improve our understanding of what a Lewy body really is. This may provide novel insight into the underlying pathological process leading to the formation of such structures, and inform on possible targets for therapeutic intervention.
The new findings here are not without controversy, as they are suggesting major differences compared with what we knew about Lewy bodies. And whenever we find the unexpected, things may become controversial. It will be important to continue to investigate the formation and composition of Lewy bodies using novel techniques, so that we develop a deeper understanding of the biological relevance of these structures.
In any case, these findings are exciting and should, if nothing else, make us rethink what we have accepted for the past 30 years.
U.K. Dementia Research Institute at University College London
Both these studies are very interesting and will propel the field forward in enabling us to better model the human disease.
For quite some time we’ve had a plethora of studies in model systems indicating aberrant lipid interaction of α-Syn as a clear mode of toxicity in the pathogenic mechanism of synucleinopathies. Their relation to Lewy body formation has been less clear. On the other hand, amyloid fibril formation has been hard to link to cellular toxicity, but had a clear link to the human pathology.
By demonstrating that lipid dysfunction, but not necessarily amyloid formation, is the actual hallmark of the disease, these studies might reconcile past findings and refocus our research more on α-Syn lipid interaction and less on amyloid aggregation, which could be quite revolutionary for the field.
That said, we need to validate these finding first in bigger cohorts, given that we are revising decades of research.
Rush University
This is an excellent paper. The field may come full circle. Phospholipids were identified as component of Lewy bodies in 1969 by histochemistry (den Jager, 1969), prior to the identification of α-synuclein.
References:
den Jager WA. Sphingomyelin in Lewy inclusion bodies in Parkinson's disease. Arch Neurol. 1969 Dec;21(6):615-9. PubMed.
Make a Comment
To make a comment you must login or register.