Do Brain Changes at Menopause Make Women More Prone to Alzheimer’s?
Quick Links
Part 1 of 2. Click here for Part 2.
More women have Alzheimer’s than men—but why? At the Alzheimer’s Association International Conference, held July 22–26 in Chicago, speakers agreed that it is not simply because women live longer, but indeed reflects a biological vulnerability. Recent epidemiological data found that women succumb to the disease at younger ages than men do, with higher risk in their late 50s and 60s, but no difference in lifetime AD risk. Scientists reported that women carry a higher tangle burden than do men with the same amyloid load, suggesting that one potential reason for their early onset might lie in a greater vulnerability to tau pathology. This association was primarily driven by APOE4 carriers. Other researchers blamed changes at menopause, when the brain’s glucose use drops. One provocative talk claimed that female brains begin to cannibalize their own myelin for energy during this time. APOE4 carriers appear most vulnerable to this disruption, perhaps leaving their brains marked for future degeneration.
- Women develop more tau pathology than men at the same amyloid burden.
- In some women, menopause causes an energy crisis in the brain that precipitates degeneration.
- ApoE4 carriers appear most vulnerable.
If that sounds disturbing, women can take solace in a bit of good news: New studies of hormone replacement therapy found that newly postmenopausal women indeed can safely take several formulations without harming their cognition. This may reassure women who have been wondering what to do after a much-ballyhooed report in 2003 about ERT and dementia risk in older women scared doctors, women—and funders, for that matter—away from this treatment for some years (May 2003 news). For more on these findings, and other epidemiological data on women, estrogen, and AD risk, read Part 2 of this story.
Overall, scientists said, new data are painting a more nuanced picture of women’s AD risk by tying it to other contributing factors. “Evidence is building that women are at increased risk for neurodegeneration in the face of risk factors such as APOE4 and high amyloid burden,” said Rachel Buckley of Massachusetts General Hospital, Boston. In other words, the vulnerability lies downstream of amyloid accumulation, she noted.
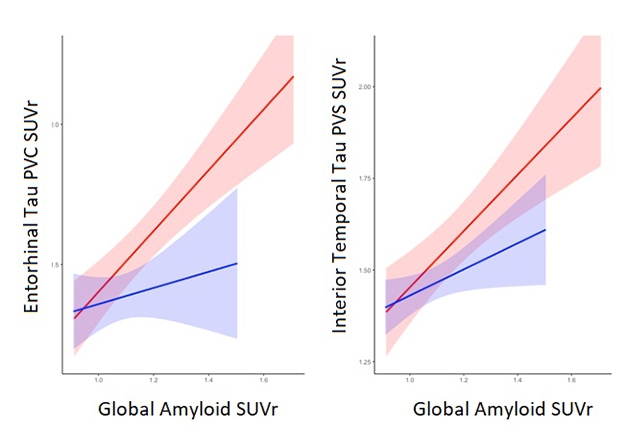
Sex Difference Opens Up At Tau Stage. As brain amyloid load increases, regional tau tangle deposition rises faster in women (red) than in men (blue). [Courtesy of Rachel Buckley.]
Two-thirds of AD patients are women, leading to the commonly held assumption that women run a higher risk of dementia. In Chicago, Arthur Toga of the University of Southern California, Los Angeles, challenged that view with data gleaned from a meta-analysis of 58,000 participants in 27 AD studies. Probing data on the Alzheimer Association’s GAAIN data-sharing platform, Toga and colleagues found no difference in AD risk between men and women overall. That said, women did run a higher risk of developing mild cognitive impairment between the ages of 55 and 70, and AD between 65 and 75. In particular, female E4 carriers were at higher risk than male carriers in this age range (Sep 2017 news).
Other talks drilled down to what might be happening in female APOE4 brains. Buckley noted that cognition declines faster in women than in men who have the same amyloid burden (Buckley et al., 2018). Since tau correlates more closely with cognitive loss than amyloid does, she wondered if tau pathology might explain this. Buckley and colleagues analyzed amyloid and tau PET scans from ADNI and the Harvard Aging Brain Study. The ADNI data comprised 103 cognitively normal controls and 58 people with MCI, while the HABS data set contained 193 cognitively normal older volunteers.
In amyloid-negative cognitively normal people, the researchers saw no sex difference in tau burden. In amyloid-positive people, however, women had more tau signal in their entorhinal cortices than men did, and that gender gap grew wider at higher amyloid burdens (see image above). Similarly, women with MCI had more tau tangles in the inferior temporal lobe than men with MCI did. Notably, the presence of an APOE4 allele boosted a woman’s tau burden more than a man’s, in keeping with Toga’s epidemiological analysis showing higher risk in female E4s. Buckley noted that a larger cohort might well reveal a more dramatic interaction between sex and APOE4.
CSF data reinforced these imaging findings. Previous cross-sectional studies have found that female APOE4 carriers have higher total and phosphorylated tau in cerebrospinal fluid than do men with similar amyloid burdens (Altmann et al., 2014; May 2018 news). Bernard Hanseeuw, also of MGH, wanted to know if tau pathology rises faster in female APOE4 carriers. He analyzed longitudinal CSF samples from an ADNI data set of 239 cognitively normal participants who had provided an average of one sample per year for three years.
In Chicago, Hanseeuw reported that across the whole cohort, total tau (t-tau) and phospho-tau 181 (p-tau) accumulated at the same rate in men and women, as well as in E4 carriers and noncarriers. However, in participants who had brain amyloid, as judged by low CSF Aβ42, p-tau rose faster in female APOE4 carriers than it did in noncarriers and male E4 carriers. This shows that sex, genotype, and pathology interact in ways worth elucidating mechanistically, Hanseeuw said.
Buckley, who collaborated with Hanseeuw, noted that this is the first data showing that tau changes faster in some cognitively normal women than men. She hopes to replicate the finding with tau PET once more longitudinal scans become available. However, Buckley and Hanseeuw both pointed out another possible explanation for more neurofibrillary tangles in women. It could be that men with that amount of tau burden are more cognitively impaired, and are thus not present in studies enrolling cognitively normal people. Men have a higher risk of cardiovascular disease, and vascular cognitive impairment could result in faster cognitive decline, Hanseeuw suggested. Further research should examine this possibility, he added.
Hanseeuw concluded that the current body of data from biomarker and epidemiological studies suggest that women who are at risk for AD, based on APOE4 genotype and amyloid accumulation, accumulate tau tangles faster than at-risk men do. In the absence of amyloid plaques, sex and genotype make no difference. Why tau pathology takes off in at-risk women remains unclear, however. “Further work in the field is important to take into account potential hormonal effects,” Hanseeuw wrote to Alzforum.
Roberta Brinton, now at the University of Arizona, Tucson, reported on one such hormonal study in Chicago. She started out by reminding the audience that the female brain undergoes a massive transition during perimenopause, when estrogen levels plummet. This hormone exerts wide-ranging effects on the brain’s metabolism, including helping it soak up glucose from blood (Apr 2015 news; Nov 2011 news). Studying aging female mice and rats, Brinton found that as their hormone levels dropped, their brains turned to ketone bodies as an alternate fuel source. Astrocytes make ketone bodies from fatty acids. To obtain enough fatty acids, the menopausal brain begins ransacking its own myelin, converting it first to lipid droplets, then to ceramides and fatty acids, Brinton said. In the brains of perimenopausal mice, she sees evidence of disorganized, disrupted myelin sheaths, along with an increase in lipid droplets (Klosinski et al., 2015; Yin et al., 2015).
Does such scary plundering happen in people? Brinton believes it does. In Chicago, she reported seeing evidence for this in a study of 43 cognitively normal women between the ages of 40 and 60, plus 18 men as controls. Women who were going through perimenopause, or were postmenopausal, had about a quarter less brain glucose metabolism, as measured by FDG PET, than premenopausal women did. They also had less active mitochondria, as measured in platelets from blood.
But the biggest change occurred in white matter, as measured by MRI. There, postmenopausal women were a whopping standard deviation below the average value in this cohort. Notably, the drops in metabolism and in white matter occurred in the regions that are affected first in AD, such as the temporal cortex and precuneus (Mosconi et al., 2017; Mosconi et al., 2017).
Brinton noted that this sample was too small to quantitatively parse out APOE4 effects. Even so, just looking at the scans, APOE4 carriers appeared to have the greatest loss of white matter. Menopausal APOE4 carriers also had more amyloid plaques than noncarriers and premenopausal women. Overall, the changes in E4 carriers mimicked those seen in the earliest stages of AD, suggesting their brains might be vulnerable to degeneration. “This could be the initiation of a prodromal phase,” Brinton said.
In ongoing research, Brinton is further examining the E4 effect on white matter in a larger cohort of perimenopausal women she follows. “We’re focused on the impact of the APOE4 genotype on the endocrine aging transition,” Brinton told Alzforum. Besides brain changes, she is also measuring the concentration of ketone bodies in blood samples from this cohort, to try to determine if human brains switch to using this form of energy at menopause as rodent brains do.
Buckley found these data on menopausal changes intriguing and thinks they could explain the differences in tau pathology she sees between older men and women. In cell culture, for example, estrogen levels have been found to modulate the kinases that induce tau hyperphosphorylation. Buckley also speculated that because APOE affects lipid metabolism, the E4 variant may lead to problems handling a potential switch to ketone body metabolism in the postmenopausal brain. She plans to test this hypothesis.
Other evidence further supports the idea that APOE4 carriers are particularly susceptible to deterioration during perimenopause. Brinton noted that in the Early versus Late Intervention Trial with Estradiol (ELITE), postmenopausal women whose metabolic health was worse also performed worse on tests of global cognition, executive function, and memory than did those whose metabolic markers were optimal. For verbal memory, the difference was significant across the cohort, though for the other cognitive tests the difference was significant only in APOE4 carriers. The metabolic markers included measures of blood glucose, cholesterol, triglycerides, ketones, and blood pressure. All of the women were within a predefined normal range on these markers, but those who performed poorly on cognitive tests were on the less-healthy end (Rettberg et al., 2016; Karim et al., 2018). The findings imply that APOE4 carriers with borderline unhealthy metabolism are at the greatest risk for AD-like changes during perimenopause, Brinton said.
Intriguingly, other new research is linking metabolism to white matter. In the August 21 JAMA Neurology, researchers in the U.K. reported that optimal metabolic and cardiovascular health associates with fewer white-matter hyperintensities and better cerebral blood flow in young adults (Williamson et al., 2018). Meanwhile, French researchers report in the same issue that having optimal metabolic markers lowered the risk of developing dementia in people older than 65 (Samieri et al., 2018). These kinds of findings are hopeful, Brinton noted, saying “You can change your metabolic phenotype.” In her perimenopausal cohort, she is investigating whether women with better metabolic health maintain thicker white matter.
Would estrogen therapy help maintain cognition? In a small trial, Brinton and Lon Schneider at the University of Southern California, Los Angeles, gave plant-based estrogen analogs called phytoSERMs or placebo for three months to a cohort of 70 postmenopausal women (Dec 2014 conference news). In this trial, women on drug had fewer hot flashes, and performed slightly better on a complex cognitive task than women on placebo, Brinton said. APOE4 carriers had the largest response to this intervention, she noted. Hot flashes have been linked to white-matter damage (Thurston et al., 2016). Brinton warned against using commercial phytoSERM preparations, however; many are made with soy extract that contains estrogen receptor antagonists, and can actually harm cognition.
Why does estrogen provide so little cognitive benefit to postmenopausal women? Brinton noted that menopause marks the end of the process of disconnecting the brain’s estrogen regulation system. Once that transition has occurred, estrogen will have little effect on brain processes. The best time for hormone replacement therapy might be during perimenopause, Brinton suggested. She is currently testing that hypothesis in perimenopausal rats. Brinton also believes that hormone therapy might be most beneficial in women who are at heightened risk for AD, namely APOE4 carriers with unhealthy metabolism.
In toto, the data highlight a growing realization that not all cases of AD have the exact same etiology, Brinton said. Factors like sex, genetics, and lifestyle can influence how AD develops during aging, and may require different interventions. “With data analytics, we can now parse out different populations of AD patients. We are at the tipping point for a precision medicine, systems biology approach to AD,” Brinton said.—Madolyn Bowman Rogers
References
News Citations
- Estrogen’s Benefit Tied to Age: Good for the Young, Bad for the Old
- Dementia Risk Increases, at Least in Those Who Start Hormone Therapy Late
- New Look at Sex and ApoE4 Puts Women at Risk Earlier than Men
- Study Finds Sex Influences CSF Tau Levels in ApoE4 Carriers
- Estrogen-like Receptor: The Gas Pedal of the Brain?
- DC: Does Estrogen Fine-Tune the Brain?
- Just for Her? Study of Women’s Biology Offers New Therapeutic Angle
Paper Citations
- Buckley RF, Mormino EC, Amariglio RE, Properzi MJ, Rabin JS, Lim YY, Papp KV, Jacobs HI, Burnham S, Hanseeuw BJ, Doré V, Dobson A, Masters CL, Waller M, Rowe CC, Maruff P, Donohue MC, Rentz DM, Kirn D, Hedden T, Chhatwal J, Schultz AP, Johnson KA, Villemagne VL, Sperling RA, Alzheimer's Disease Neuroimaging Initiative, Australian Imaging, Biomarker and Lifestyle study of ageing, Harvard Aging Brain Study. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer's disease: Findings from three well-characterized cohorts. Alzheimers Dement. 2018 Sep;14(9):1193-1203. Epub 2018 May 24 PubMed.
- Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer's Disease Neuroimaging Initiative Investigators. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014 Apr;75(4):563-73. Epub 2014 Apr 14 PubMed.
- Klosinski LP, Yao J, Yin F, Fonteh AN, Harrington MG, Christensen TA, Trushina E, Brinton RD. White Matter Lipids as a Ketogenic Fuel Supply in Aging Female Brain: Implications for Alzheimer's Disease. EBioMedicine. 2015 Dec;2(12):1888-904. Epub 2015 Nov 3 PubMed.
- Yin F, Yao J, Sancheti H, Feng T, Melcangi RC, Morgan TE, Finch CE, Pike CJ, Mack WJ, Cadenas E, Brinton RD. The perimenopausal aging transition in the female rat brain: decline in bioenergetic systems and synaptic plasticity. Neurobiol Aging. 2015 Jul;36(7):2282-2295. Epub 2015 Apr 1 PubMed.
- Mosconi L, Berti V, Guyara-Quinn C, McHugh P, Petrongolo G, Osorio RS, Connaughty C, Pupi A, Vallabhajosula S, Isaacson RS, de Leon MJ, Swerdlow RH, Brinton RD. Perimenopause and emergence of an Alzheimer's bioenergetic phenotype in brain and periphery. PLoS One. 2017;12(10):e0185926. Epub 2017 Oct 10 PubMed.
- Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Varsavsky I, Osorio RS, Pupi A, Vallabhajosula S, Isaacson RS, de Leon MJ, Brinton RD. Sex differences in Alzheimer risk: Brain imaging of endocrine vs chronologic aging. Neurology. 2017 Sep 26;89(13):1382-1390. Epub 2017 Aug 30 PubMed.
- Rettberg JR, Dang H, Hodis HN, Henderson VW, St John JA, Mack WJ, Brinton RD. Identifying postmenopausal women at risk for cognitive decline within a healthy cohort using a panel of clinical metabolic indicators: potential for detecting an at-Alzheimer's risk metabolic phenotype. Neurobiol Aging. 2016 Apr;40:155-63. Epub 2016 Jan 29 PubMed.
- Karim R, Koc M, Rettberg JR, Hodis HN, Henderson VW, St John JA, Allayee H, Brinton RD, Mack WJ. Apolipoprotein E4 genotype in combination with poor metabolic profile is associated with reduced cognitive performance in healthy postmenopausal women: implications for late onset Alzheimer's disease. Menopause. 2019 Jan;26(1):7-15. PubMed.
- Williamson W, Lewandowski AJ, Forkert ND, Griffanti L, Okell TW, Betts J, Boardman H, Siepmann T, McKean D, Huckstep O, Francis JM, Neubauer S, Phellan R, Jenkinson M, Doherty A, Dawes H, Frangou E, Malamateniou C, Foster C, Leeson P. Association of Cardiovascular Risk Factors With MRI Indices of Cerebrovascular Structure and Function and White Matter Hyperintensities in Young Adults. JAMA. 2018 Aug 21;320(7):665-673. PubMed.
- Samieri C, Perier MC, Gaye B, Proust-Lima C, Helmer C, Dartigues JF, Berr C, Tzourio C, Empana JP. Association of Cardiovascular Health Level in Older Age With Cognitive Decline and Incident Dementia. JAMA. 2018 Aug 21;320(7):657-664. PubMed.
- Thurston RC, Aizenstein HJ, Derby CA, Sejdić E, Maki PM. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016 Jan;23(1):27-32. PubMed.
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.