Head-to-Head Study Confirms Plasma p-Tau231 Rises First in Early AD
Quick Links
How early in the course of Alzheimer’s disease can a blood marker detect amyloid pathology, and which marker does it best? At this year’s Alzheimer’s Association International Conference, held from July 31 to August 4 in San Diego, California, and online, presenters converged on the same conclusion after comparing markers head to head: Plasma p-tau231 rises soonest after Aβ42/40 begins to fall in cerebrospinal fluid or after the locus coeruleus begins to atrophy. On the other hand, p-tau217 and p-tau181 tick up later, but they better track with amyloid plaques, neurofibrillary tangles, and cognitive decline. “With different p-tau forms changing at different time points during the AD process, there is the (theoretical) possibility of staging patients, but more studies are needed to confirm if this indeed is useful,” Henrik Zetterberg of the University of Gothenburg, Sweden, told Alzforum. People with multiple p-tau isoforms in their CSF also had more amyloid, tangles, and cognitive decline than those who tested positive for just one isoform.
- In cognitively normal adults, plasma p-tau231 rises as Aβ42/40 drops in CSF.
- But plasma p-tau217 and p-tau181 better track cognitive decline.
- Plasma p-tau231 also indicates atrophy of the locus coeruleus.
- The number of p-tau isoforms in CSF better correlates with plaques, tangles, and cognitive decline than do single isoforms.
Nicholas Ashton, also at UGot, previously reported that p-tau231 rises in the blood at the first signs of plaques on amyloid PET scans and that it predicts worsening on the Mini-Mental State Examination over one year (Feb 2021 news). At last year’s AAIC, he reported that plasma p-tau231 also rose when the Aβ42/40 ratio in the CSF fell, which happens years before plaques can be detected by PET and decades before cognitive symptoms (Aug 2021 conference news). But the marker had never been directly compared with other p-tau fragments in the blood, nor measured in many normal older adults. How would the markers compare, especially among cognitively normal people who might be on the cusp of early AD pathology?
Ashton and colleagues, led by Kaj Blennow of UGot and Marc Suárez-Calvet, Barcelonaβeta Brain Research Center, Spain, expanded their analyses to include the three most promising p-tau markers—181, 217, and 231—testing them in a large cohort of older, cognitively normal adults who are at risk for AD.
Ashton and Marta Milà-Alomà of Barcelonaβeta focused on 397 volunteers in the ALFA+ cohort, which comprises middle-aged volunteers who have normal cognition but a family history of dementia. One hundred thirty-five tested amyloid-positive by their CSF Aβ42/40. Of 339 volunteers who had an amyloid PET scan, 53 had signs of Aβ aggregation, having a Centiloid score above 12, while 26 of those were deemed to have established amyloid plaque pathology with a score above 30. A subset of 214 participants had taken the Preclinical Alzheimer Cognitive Composite test at baseline and at an average of three years later.
Because they had only cross-sectional data, the researchers used amyloid burden as a proxy for disease progression. As expected, p-tau231 was the first plasma p-tau marker to increase. Whether correlated to CSF Aβ42/40 or amyloid PET, it reached a concentration two standard deviations higher than that in amyloid-negative people before any other marker (see image below). P-tau217 rose next, followed by glial fibrillary acidic protein (GFAP), a marker of astrocytosis. Neither p-tau181 nor the neurodegeneration marker NfL crossed the 2-standard-deviation threshold. The researchers concluded that p-tau231 is the earliest p-tau marker of amyloidosis.

Early Riser. Using CSF Aβ42/40 (left) or Centiloids (right) as a proxy for disease progression (yellow), plasma p-tau231 (orange) ticks up earliest in AD. It is also the first to become abnormal, as defined by the marker level being two standard deviations above concentrations in people with normal CSF Aβ42/40, i.e. a ratio greater than 0.10. [Courtesy of Milà-Alomà et al., Nature Medicine, 2022.]
P-tau231 also had the tightest correlation with CSF amyloid positivity after the scientists adjusted for age, sex, and APOE4 status, with an area under the curve of 0.81. AUC is a statistical measure of sensitivity and specificity, with 1.0 being perfection.
“This work builds on the existing literature to understand the temporality of the blood-based AD-related biomarkers among middle-aged adults,” wrote Michelle Mielke, Wake Forest University, Winston-Salem, North Carolina (full comment below). Ashton and colleagues published their results in the August 11 Nature Medicine (Milà-Alomà et al., 2022).
What about cognitive decline? In her poster, Milà-Alomà showed that amyloid-positive people who had high p-tau231 or p-tau181, but not high p-tau217, in their plasma at baseline did worse on the PACC three years later. Among amyloid-negative participants there was no correlation with any baseline p-tau fragment and the PACC, although cognitive scores did trend down in people who had high baseline p-tau181. Indeed, considering both amyloid positives and negatives, Milà-Alomà found that the relationship between PACC change and plasma p-tau was driven by amyloid status only for p-tau231 (see image below). This marker, specifically, seems to associate with preclinical cognitive decline driven by early amyloid pathology, Milà-Alomà told Alzforum.
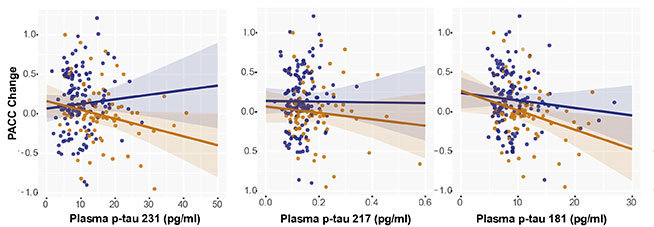
P-tau and PACC. In cognitively normal adults who tested positive for amyloid in CSF analysis (tan), high baseline plasma p-tau231 (left) and high p-tau181 (right) predicted decline on the PACC over three years. In amyloid-negative adults, there was no correlation (blue). Ditto for either group with p-tau217 (middle). [Courtesy of Marta Milà-Alomà, Barcelonaβeta Brain Research Center, Spain.]
Next, Milà-Alomà focused on 33 ALFA+ participants whose PACC score had slipped the most. In these, high baseline plasma p-tau181 and p-tau217—but not p-tau231—correlated with PACC change. Why not p-tau231? Though the small sample might be unreliable, Milà-Alomà hypothesized that the decliners had worsened to a disease stage where p-tau231 was less predictive than the other p-tau markers. This would be in keeping with p-tau231 rising then reaching a plateau before the other tau isoforms. She is now correlating how the plasma markers change over time with cognition.
Ashton did just that in two other cohorts, measuring the longitudinal change in plasma markers and MMSE scores. Oskar Hansson of Lund University, Sweden, presented the results at AAIC. The scientists, including Shorena Janelidze and Niklas Mattsson-Carlgren at Lund, measured the same six plasma markers—p-tau181, 217, and 231, and Aβ42/40, GFAP, and NfL—at baseline and an average of three times over six years among people who were cognitively normal or had signs of dementia at baseline. They analyzed samples from 388 cognitively normal participants and 187 people with mild cognitive impairment in the Swedish BioFINDER cohort and from 161 cognitively unimpaired participants from the Wisconsin Registry for Alzheimer’s Prevention. WRAP participants are at risk of developing AD because at least one parent had the disease.
First, the researchers divided participants into five groups based on baseline amyloid PET. Once again, the baseline data hinted that p-tau231 rises first in AD. This marker was high among people who clocked in with as few as 12 Centiloids, while p-tau217 or p-tau181 did not budge until Centiloid levels hit 36.
What about longitudinal change? Over the six years, p-tau217 rose the most of all the isoforms in amyloid-positive, cognitively normal people and in those with MCI who were amyloid-positive. The increase associated with accelerated cortical thinning and worse decline in MMSE scores (see image below). Again, why no correlation with p-tau231? Because it plateaued after early amyloid accumulation, while p-tau217 continuously rose with plaque load, Hansson noted.

P-tau217 Tracks Change. Compared to plasma p-tau231 (left) and p-tau181 (right), p-tau217 (middle) rose more in PET amyloid-positive (blue) versus negative (gray) cognitively unimpaired people (top row). In the amyloid-positive participants, cortical atrophy (middle row) and cognition (bottom row) worsened faster in people who had the highest plasma p-tau217 change (pink) than in those with the average (turquoise) or less change (purple). [Courtesy of Oskar Hansson, Lund University, Sweden.]
All told, both ALFA+ and BioFINDER/WRAP studies support the idea that plasma p-tau231 is the earliest isoform to detect amyloidosis in cognitively normal people, while p-tau217 and 181 are better predictors of longitudinal cognitive decline.
This held in yet more cohorts. At AAIC, Pamela Ferreira from the University of Pittsburgh reported that, compared to Aβ42/40, GFAP, and NfL, p-tau231 best indicated who among 138 cognitively normal volunteers in the Translational Biomarkers of Aging and Dementia (TRIAD) cohort in Montreal, Canada, were amyloid-positive by PET. The AUC was 0.85. Ferreira did not measure p-tau217 or p-tau181. Further, Hansson recently reported that among people with Down's syndrome, plasma p-tau217 correlates with plaques, tangles, and faltering cognition in people who do not yet have dementia (Jul 2022 news).
P-tau Predicts Atrophy, Degree of Pathology
Can plasma p-tau predict more about the brain than amyloid positivity? Milà-Alomà and Ashton correlated various tau isoforms in the plasma with the accumulation of plaques in different brain regions. They found that plasma p-tau231 and p-tau217 most strongly associated with the earliest PET signals, including those in the orbitofrontal cortices, cingulate gyri, insula, and precuneus.
Similarly, Heidi Jacobs, Massachusetts General Hospital, Boston, tied p-tau231 to early brain atrophy. She correlated plasma p-tau231, p-tau217, p-tau181, total tau, Aβ42/40, and NfL with high resolution 7T structural MRI scans and PACC scores from 99 cognitively normal people ages 30 to 85 in an MRI cohort study based at Maastricht University in the Netherlands (Van Egroo et al., 2021).
As in the ALFA+ cohort, Jacobs found that high plasma p-tau231 correlated with low PACC scores. From the MRI scans, she could see that the marker also tracked with atrophy in the locus coeruleus, picking up withering there in people as young as 55. This tiny area within the brain stem is one of the first sites to accumulate hyperphosphorylated tau in AD, and it shrinks 8.4 percent with each Braak stage (Braak et al., 2011; Theofilias et al., 2016). “These volumetric changes reflect the accumulation of tau pathology and loss of neuronal projections to the cortex, which may impact how the locus coeruleus communicates with cortical brain systems,” Jacobs said during her presentation.
Stijn Servaes of McGill University in Canada took a different approach to studying the various tau isoforms, asking what, if anything, the number of different p-tau isoforms in the CSF might reveal. First, using amyloid PET and CSF data from 60 amyloid-positive and 107 amyloid-negative TRIAD participants, he ran a machine-learning algorithm to establish cutoffs for amyloid positivity for different p-tau isoforms. Next, Servaes used those cutoffs to ask how the number of positive p-tau isoforms relates to plaques, tangles, and cognition.
Among 59 people with MCI or AD, and 73 age-matched controls, Servaes found that the more CSF p-tau species a person tested positive for, the higher their plaque and tangle load and the lower their MMSE score. People positive for two or more p-tau isoforms were amyloid-positive. Those positive for three different p-tau isoforms also tested positive for tangles. Finally, people with four p-tau species in their CSF had MMSE scores of 28 or less. The findings suggest that the different p-tau isoforms reflect cumulative damage in the brain.
Scientists hope to capitalize on these latest findings to develop better markers for AD diagnosis and staging. Researchers could choose which marker to measure based on which stage of preclinical AD they want to study, Suárez-Calvet said. For example, selecting people with high plasma p-tau231 would enrich for those who are just beginning to accumulate amyloid, while selecting for high p-tau217 would target those farther in the clinical trajectory at the threshold of robust plaque deposition. However, Mielke does not think it is that straightforward. “We are not at the point yet to determine what p-tau species should be used at each specific stage of the disease, either for diagnosis or prognosis of AD,” she cautioned.
This is, in part, because plasma assays are not yet reproducible or standardized (Oct 2021 news; Mielke et al., 2021). “[This is] a major problem in the field right now,” wrote Suzanne Schindler at Washington University in St. Louis. “It is difficult to conclude which analyte is best without considering assay performance. For example, it is possible that plasma Aβ42/40 changes before p-tau231, and the “earlier” changes in p-tau231 are artifactual because the p-tau231 assay is more sensitive or precise than the Aβ42/40 assay,” she added (comment below). For more on plasma marker development, see Part 4 of this series.—Chelsea Weidman Burke
References
News Citations
- Earliest of Them All: Blood P-Tau231 Assay Flags Pre-Amyloid Alzheimer’s
- Mirror, Mirror on the Wall, Who’s the Earliest of Them All?
- In Down's Syndrome, Blood P-Tau217 Detects Plaques and Tangles
- In Side-by-Side Test of 8 Blood Aβ Assays, Mass Spec Shines
- Alzheimer's Blood Tests Have Arrived; Road to Broad Use Still Stretches On
Paper Citations
- Milà-Alomà M, Ashton NJ, Shekari M, Salvadó G, Ortiz-Romero P, Montoliu-Gaya L, Benedet AL, Karikari TK, Lantero-Rodriguez J, Vanmechelen E, Day TA, González-Escalante A, Sánchez-Benavides G, Minguillon C, Fauria K, Molinuevo JL, Dage JL, Zetterberg H, Gispert JD, Suárez-Calvet M, Blennow K. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer's disease. Nat Med. 2022 Sep;28(9):1797-1801. Epub 2022 Aug 11 PubMed. Correction.
- Van Egroo M, van Hooren RW, Jacobs HI. Associations between locus coeruleus integrity and nocturnal awakenings in the context of Alzheimer's disease plasma biomarkers: a 7T MRI study. Alzheimers Res Ther. 2021 Sep 24;13(1):159. PubMed.
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011 Nov;70(11):960-9. PubMed.
- Theofilas P, Ehrenberg AJ, Dunlop S, Di Lorenzo Alho AT, Nguy A, Leite RE, Rodriguez RD, Mejia MB, Suemoto CK, Ferretti-Rebustini RE, Polichiso L, Nascimento CF, Seeley WW, Nitrini R, Pasqualucci CA, Jacob Filho W, Rueb U, Neuhaus J, Heinsen H, Grinberg LT. Locus coeruleus volume and cell population changes during Alzheimer's disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement. 2016 Aug 8; PubMed.
- Mielke MM, Frank RD, Dage JL, Jeromin A, Ashton NJ, Blennow K, Karikari TK, Vanmechelen E, Zetterberg H, Algeciras-Schimnich A, Knopman DS, Lowe V, Bu G, Vemuri P, Graff-Radford J, Jack CR Jr, Petersen RC. Comparison of Plasma Phosphorylated Tau Species With Amyloid and Tau Positron Emission Tomography, Neurodegeneration, Vascular Pathology, and Cognitive Outcomes. JAMA Neurol. 2021 Sep 1;78(9):1108-1117. PubMed.
External Citations
Further Reading
Primary Papers
- Milà-Alomà M, Ashton NJ, Shekari M, Salvadó G, Ortiz-Romero P, Montoliu-Gaya L, Benedet AL, Karikari TK, Lantero-Rodriguez J, Vanmechelen E, Day TA, González-Escalante A, Sánchez-Benavides G, Minguillon C, Fauria K, Molinuevo JL, Dage JL, Zetterberg H, Gispert JD, Suárez-Calvet M, Blennow K. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer's disease. Nat Med. 2022 Sep;28(9):1797-1801. Epub 2022 Aug 11 PubMed. Correction.
Annotate
To make an annotation you must Login or Register.

Comments
Mayo Clinic
In this study, Milà-Alomà et al. compared the ability of different plasma biomarkers to identify Alzheimer’s pathology at a very early stage in cognitively normal individuals from the ALFA+ cohort. The main objective of the study was to test whether plasma p-tau231, a newer p-tau proteoform, becomes abnormal in early preclinical AD, and to compare its ability to detect Aβ pathology at a very early stage with other plasma biomarkers (p-tau 217, p-tau181, GFAP and neurofilament light). This is an important and highly timely study.
The head-to-head comparison with other plasma biomarkers indicated that p-tau231 is an early changing plasma analyte, comparable to p-tau217 and perhaps even earlier changing than it. As the authors indicate, the ability to confidently detect emerging AD pathology in unimpaired individuals is important now, and will become more so as therapeutic intervention trials move to increasingly earlier stages in the disease. This study brings p-tau231 to the fore as a promising analyte that should be included in future head-to-head studies comparing the diagnostic performance of various plasma analytes in the early phase of preclinical AD.
Mayo Clinic
The paper by Mila-Aloma and colleagues nicely builds on the existing literature to understand the temporality of the blood-based AD-related biomarkers among middle-aged adults. P-tau231 appears to change first, followed closely by p-tau217 and then other markers. The population is relatively young with a high risk of developing AD, so the generalizability of the results to older populations remains unclear and additional research will be needed. In addition, p-tau217 and p-tau231 were measured on different platforms and it is a bit unclear what role that aspect played in the observed differences versus the differences in isoforms. The authors appropriately discuss these aspects in the paper.
Overall, I think the fielding is rapidly moving forward and it is a really exciting time. We are not at the point yet to determine exactly what p-tau species should be used at each specific stage of the disease—either for diagnosis or prognosis of AD—but additional information is accumulating.
Washington University
Milá-Alomá et al. evaluated plasma biomarkers in the ALFA+ cohort to see which captured early changes in amyloid accumulation. They report that plasma p-tau231 reached abnormal levels with the lowest amyloid burden. However, a key caveat to consider is that different assays have very different performance, which makes comparisons across analytes challenging. For example, it is possible that plasma Aβ42/40 changes before p-tau231, and the “earlier” changes in p-tau231 seen in this analysis are artifactual because the p-tau231 assay is more sensitive/precise than the Aβ42/40 assay. This is a particular issue because abnormality was defined as being 2 standard deviations from the mean, such that a less precise assay would lead to abnormal measurements being considered normal because of the higher variance of the assay. The Cohen’s D measures described would also be susceptible to differences in assay performance, as the denominator is essentially the variance of measures.
The authors did evaluate the performance of plasma biomarkers in prediction of CSF Aβ42/40 status, although the ROC AUCs for all analytes were lower (AUC 0.75) than is often reported (AUC 0.80-0.90). This suggests that assay performance could indeed be a significant factor affecting the results and conclusions. A continuous non-parametric analysis (e.g., Spearman) of the plasma biomarkers versus CSF Aβ42/40 and amyloid PET centiloid would also be helpful in evaluating the relative performance of the assays.
It is clear that multiple plasma biomarkers change very early in the course of AD. This reinforces the idea that plasma biomarkers are extremely useful tools in clinical trials and the clinic. Additional analyses of longitudinal plasma biomarkers that consider the effects of assay performance (or use approaches less affected by assay performance) are needed before making any firm conclusions about which plasma measures change first.
VU University Medical Center
The Ashton et al. paper adds to the rapid growth of information on plasma p-tau tests and other blood tests for Alzheimer’s disease. The inclusion of a large number of people with early stage AD pathology and the direct comparison of the performance of many markers is very informative. The data show that changes in early stages of amyloid pathology are observed for all studied markers—p-tau181, p-tau217, p-tau213, Aβ42/40 ratio, GFAP, and NfL—measured on different platforms (Cobas, Simoa, MSD). Modelled p-tau217 and p-tau213 increases were steeper and might thus be slightly more sensitive to detect early changes in amyloid load.
However, the authors showed different results depending on the outcomes. In early A+T- pathology versus A-T- controls, the Aβ42/40 ratio, plasma p-tau231, and p-tau217 appeared the more sensitive markers, while in A+T+ versus A-T-, plasma p-tau231 had the largest effect size. Using area under the curve analysis to discriminate Aβ status (defined by CSF Aβ42/40 ratio), all markers performed equally well, adding to the base model, which included the risk factors age, sex, and APOE status, with overlapping confidence intervals, though here, the p-tau231 marker did not survive multiple comparisons. This shows that differences in performances are small between all markers, and that all have added value for early biological diagnosis of AD.
These results align well with published studies in which there are subtle differences between different p-tau isoform tests (e.g., Thijssen et al., 2021, where the pTau181 and pTau217 tests conditions were identical except for the nature of the primary antibody; and Bayoumy et al., 2021, which included only Simoa assays). These differences may depend on affinities of antibodies, buffers used in the immunoassays, sensitivities of the platforms, or robustness of reagents. It is also interesting that the Aβ42/40 ratio, which shows only a small absolute difference between A+ and A- (12 percent) still shows a similar effect size as the p-tau assays, which typically show larger differences. Thus, not only do absolute differences matter, but so does variation between individuals.
The large preclinical cohort is very relevant for trial inclusion for early stage AD. Since all the markers seem to combine well, the choice for one or the other combination may depend on other factors, such as ease of multiplexing, cost-effectiveness, commercial availability, and throughput.
The authors additionally showed that the optimal model depended on the chosen age group and age group cut-off (61.8 versus 65 years). This warrants future investigation, e.g., if this affects inclusion rates and trial outcomes, since for implementation in trials, ideally one model that includes one set of markers should be used.
What is next? Now that these assays have become more widely available, the models defined by Milà-Alomà should be tested prospectively, in real-world trials. The AUCs were not perfect, due to considerable overlap in biomarker values between the different groups studied. This argues for confirmation of the cases defined as positive using CSF or PET amyloid testing, as recommended in the recently published appropriate-use recommendations. The number of tests and platforms for all these different markers are rapidly expanding, and subtle differences between platforms can also be expected, but may not be crucial for the success of a trial if results are confirmed by CSF or PET testing.
Detecting amyloid pathology (A+) and/or AD pathology (A+T+) is relevant also in clinics where plasma markers could replace other biomarker measures, but it is worth considering the follow-up questions patients may have, e.g., about clinical prognosis. In this respect the results of Milà-Alomà et al. showing prognostic value for cognitive decline for only p-tau181 in the total group, and a nominal significant interaction for p-tau231 in the patients who were A+ as measured with the CSF Aβ42/40, are interesting. The lack of effects for GFAP and NfL contradicts other studies in preclinical AD, which might again be due to differences in outcome measures, and stresses that although the field is moving fast, more research is needed to address many questions (Verberk et al., 2021).
References:
Thijssen EH, La Joie R, Strom A, Fonseca C, Iaccarino L, Wolf A, Spina S, Allen IE, Cobigo Y, Heuer H, VandeVrede L, Proctor NK, Lago AL, Baker S, Sivasankaran R, Kieloch A, Kinhikar A, Yu L, Valentin MA, Jeromin A, Zetterberg H, Hansson O, Mattsson-Carlgren N, Graham D, Blennow K, Kramer JH, Grinberg LT, Seeley WW, Rosen H, Boeve BF, Miller BL, Teunissen CE, Rabinovici GD, Rojas JC, Dage JL, Boxer AL, Advancing Research and Treatment for Frontotemporal Lobar Degeneration investigators. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer's disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021 Sep;20(9):739-752. PubMed.
Bayoumy S, Verberk IM, den Dulk B, Hussainali Z, Zwan M, van der Flier WM, Ashton NJ, Zetterberg H, Blennow K, Vanbrabant J, Stoops E, Vanmechelen E, Dage JL, Teunissen CE. Clinical and analytical comparison of six Simoa assays for plasma P-tau isoforms P-tau181, P-tau217, and P-tau231. Alzheimers Res Ther. 2021 Dec 4;13(1):198. PubMed.
Verberk IM, Laarhuis MB, van den Bosch KA, Ebenau JL, van Leeuwenstijn M, Prins ND, P, Teunissen CE, van der Flier WM. Serum markers glial fibrillary acidic protein and neurofilament light for prognosis and monitoring in cognitively normal older people: a prospective memory clinic-based cohort study. The Lancet Healthy Longevity, January 19, 2021 The Lancet Healthy Longevity
Washington University School of Medicine
At AAIC, several groups presented results on head-to-head comparisons between p-tau isoform markers. There are now numerous publications assessing the performance of the different plasma p-tau assays designed over the past three years. There is also now a consensus that most of the plasma p-tau assays have excellent accuracy to differentiate AD from controls or to identify AD pathology amongst subjects with cognitive symptoms (Bayoumy et al., 2021; Palmqvist et al., 2020). It is less clear how these assays could accurately identify AD amongst subjects without cognitive symptoms.
It is worth mentioning there are several platforms measuring the same p-tau sites. The comparisons have usually indicated different levels of performance. In other words, attributing better performance to a particular p-tau also has to be balanced by the performance of the analytical platform used. This is even more crucial in plasma, where the p-tau species are present in very low abundance in a highly complex matrix, increasing the probability of interference. Globally, the Lilly p-tau217 assay, used in many of the p-tau assay comparisons, seems to slightly outperform the others. At AAIC, Oskar Hansson reported the mass spectrometry assay we have designed at WUSTL, measuring plasma p-tau217, which could have significantly better performance than other p-tau assays, including Lilly’s p-tau217 assay.
I think that a consensus about the “best’’ plasma p-tau site could only emerge if several platforms measuring the same site would report better results than a set of platforms measuring another site.
Our group uses a mass spectrometry platform to compare the performance of 10 phosphorylated residues, including T217, T231 and T181, for the identification of participants with amyloid deposition or tau deposition in the brain as measured by PET. Our results in CSF have suggested these three phosphorylated sites increase in response to amyloid deposition. These three sites are hyperphosphorylated in preclinical AD, together with T111, T153 and S208.
P-tau231 has been already proposed as an early biomarker for brain amyloid, with a significant increase observed in CSF and plasma from participants with amyloid deposition (Ashton et al., 2021). In the present study, the authors report that the same p-tau231 Gothenburg assay, together with the Lilly p-tau217 assay, can measure significant increases of p-tau in cognitively unimpaired participants. These results are in line with accuracies reported previously by Mielke et al. to detect amyloid positivity in cognitively unimpaired subjects when comparing plasma p-tau assays, including Gothenburg p-tau231 and Lilly p-tau217 (Mielke et al., 2021).
The AUC from the p-tau assays reported for detecting preclinical AD are typically below 0.8 after inclusion of risk factors such as age, sex, and APOE status. Alone, they are not significantly better than a prediction based solely on risk factors. Though cohorts from one study to another could not be strictly compared, these performances seem lower than what was reported using plasma amyloid assays, notably by mass spectrometry (Ovod et al., 2017). These identified amyloid-positive participants in preclinical AD with an AUC of 0.88, without including risk factors (Schindler et al., 2019). Of note, the plasma Aβ test used in the Milà-Alomà paper is the Neurology 4-Plex Advantage Kit, which has not showed the best performance compared to other plasma Aβ assays (Janelidze et al., 2021). It would have been interesting to include another plasma Aβ assay for this comparison with p-tau assays.
I think the most accurate plasma assays for identifying preclinical AD remain the MS assays measuring the plasma Aβ42/40 ratio. It is indisputable that p-tau, on the sites currently investigated, is modified in AD primarily due to amyloid deposition and that these sites can be used as complement biomarkers. Thus, plasma p-tau biomarkers can serve to build clinical cohorts enriched with asymptomatic amyloid-positive participants, which will be fundamental to test preventive drugs in trials. However, the moderate accuracy of these tests will limit efficient enrichment of such targeted participants. Ultimately the use of plasma p-tau might not allow us to dispense with diagnostic confirmation using CSF biomarkers or PET imaging.
References:
Bayoumy S, Verberk IM, den Dulk B, Hussainali Z, Zwan M, van der Flier WM, Ashton NJ, Zetterberg H, Blennow K, Vanbrabant J, Stoops E, Vanmechelen E, Dage JL, Teunissen CE. Clinical and analytical comparison of six Simoa assays for plasma P-tau isoforms P-tau181, P-tau217, and P-tau231. Alzheimers Res Ther. 2021 Dec 4;13(1):198. PubMed.
Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, Su Y, Chen Y, Serrano GE, Leuzy A, Mattsson-Carlgren N, Strandberg O, Smith R, Villegas A, Sepulveda-Falla D, Chai X, Proctor NK, Beach TG, Blennow K, Dage JL, Reiman EM, Hansson O. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA. 2020 Aug 25;324(8):772-781. PubMed.
Ashton NJ, Pascoal TA, Karikari TK, Benedet AL, Lantero-Rodriguez J, Brinkmalm G, Snellman A, Schöll M, Troakes C, Hye A, Gauthier S, Vanmechelen E, Zetterberg H, Rosa-Neto P, Blennow K. Plasma p-tau231: a new biomarker for incipient Alzheimer's disease pathology. Acta Neuropathol. 2021 May;141(5):709-724. Epub 2021 Feb 14 PubMed.
Mielke MM, Frank RD, Dage JL, Jeromin A, Ashton NJ, Blennow K, Karikari TK, Vanmechelen E, Zetterberg H, Algeciras-Schimnich A, Knopman DS, Lowe V, Bu G, Vemuri P, Graff-Radford J, Jack CR Jr, Petersen RC. Comparison of Plasma Phosphorylated Tau Species With Amyloid and Tau Positron Emission Tomography, Neurodegeneration, Vascular Pathology, and Cognitive Outcomes. JAMA Neurol. 2021 Sep 1;78(9):1108-1117. PubMed.
Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, Sullivan M, Paumier K, Holtzman DM, Morris JC, Benzinger T, Fagan AM, Patterson BW, Bateman RJ. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017 Aug;13(8):841-849. Epub 2017 Jul 19 PubMed.
Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, Holtzman DM, Morris JC, Benzinger TL, Xiong C, Fagan AM, Bateman RJ. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019 Oct 22;93(17):e1647-e1659. Epub 2019 Aug 1 PubMed.
The University of Melbourne
One of the criticisms of the amyloid hypotheses has been that tau and Aβ are decoupled temporally. Now, it is not just plasma p-tau (as in Milá-Alomá et al.) but PET scans that show this relationship starts early. Using PET scans optimized for quantification rather than visual reporting, and using data-driven, deep-learning approaches guided by relative spatial positioning rather than uptake levels, we have revealed new relationships between tau topography and Aβ burden (Ruwanpathirana et al., 2022). Topography, not just amount, is critical to this relationship. It does not start late in the natural history of AD, rather it occurs at minimal or low levels of Aβ burden. The differential importance of tau-accumulation regions with the Aβ load may be considered a proxy for longitudinal change, and it may provide insight into Alzheimer’s disease progression. This method may be used to show the topographic relationship between images and other biomarkers, not just PET.
References:
Ruwanpathirana GP, Williams RC, Masters CL, Rowe CC, Johnston LA, Davey CE. Mapping the association between tau-PET and Aβ-amyloid-PET using deep learning. Sci Rep. 2022 Aug 30;12(1):14797. PubMed.
Make a Comment
To make a comment you must login or register.