
Series
Deep-Brain Stimulation: Surgical Relief for Parkinson's and Beyond
Deep-Brain Stimulation: Decade of Surgical Relief, Not Just for PD
Twelve years on, Jeff Ryan still recalls the collective gasp in the operating room the day he got the electrodes implanted. He himself was in somewhat of a state of shock as he held the cup. “All of the sudden your hands that have been shaking like crazy all your life, are just rock steady,” he recalled. The Iowan had had essential tremor since his teens, and was starting to have trouble managing his farm because of the trembling. Surgeons ran wires into his brain and hooked them up to a pacemaker-like neurostimulator implanted in his chest, which sends electrical signals to his brain that quiet the tremors. “It just changes your life dramatically,” Ryan told ARF.
It’s not just Ryan, and it’s not just tremor. On April 28 in the Lancet Neurology online, researchers from Queen Elizabeth Hospital in Birmingham, UK, report on behalf of the PD SURG Collaborative Group that deep-brain stimulation plus medication led to greater improvement in Parkinson disease symptoms than meds alone. This was a six-year, 13-center trial, the largest to date (Williams et al., 2010).
In this series, ARF takes stock of deep-brain stimulation after more than a decade of life-altering procedures. Now is an interesting time to learn more about this surgical treatment, because even as scientists are gathering long-term, broad-based data on its initial indications—movement disorders—they are beginning to explore whether DBS might also work for a range of other conditions, including Alzheimer’s.
Deep-brain stimulation (DBS for short) has been used to treat essential tremor and Parkinson disease tremors since 1997, when the FDA approved the therapy. More than 55,000 DBS devices have been implanted worldwide, many targeting the tremors and dyskinesias of Parkinson disease. Doctors say even more people could benefit from DBS. “It is underutilized, in my opinion,” said Jerrold Vitek, a movement disorder neurologist and leading DBS researcher, who co-chaired the Center for Neurological Restoration at the Cleveland Clinic until his present move to the University of Minnesota in Minneapolis-St. Paul.
Scientists are starting to gather data on the long-term effects of the treatment, on symptoms as well as quality of life. In the case of Parkinson’s, the effects of DBS can seem miraculous—but it is not a solution for all life’s ills, recipients and researchers find. Sometimes, once the tremors and dyskinesias have stopped, problems can arise in other aspects of a person’s life such as work or relationships (see Part 2 of this series).
Beyond PD, clinical trials are running for myriad targets. The specific site for DBS is based on imaging studies showing where, in a given condition, the brain is struggling. Therefore, any condition linked to altered brain activity in a particular spot is a potential candidate for DBS (see Part 3 of this series). Neurosurgeons implant tiny wires, usually carrying four electrodes each, into the appropriate brain area. In a second procedure, surgeons place a neurostimulator just beneath the skin, frequently below the collarbone. Wires under the skin connect the two implements. The neurostimulator can provide electrical signals that are strong or weak, fast or slow, depending on the person’s needs. Researchers hope to improve both the surgical process of DBS—making it safer—as well as the post-operative process of programming the neurostimulator to provide the ideal signals (see Part 4.
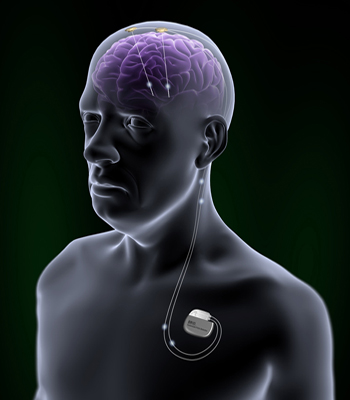
In deep-brain stimulation, a neurostimulator under the skin drives current to electrodes implanted into the brain. Image credit: St. Jude Medical
Optimizing Outcomes
In the most recent study of DBS outcomes, the Birmingham researchers and colleagues throughout the UK followed 366 people with advanced Parkinson disease (Williams et al., 2010). Half received DBS plus the best medical therapy available; the other half received only medical treatment. One year on, people who had surgery reported better mobility, ease in daily activities, and a drop in discomfort. People on the best medical therapy, in comparison, reported little change in their condition.
However, the scientists noted, DBS also led to more serious side effects, including 16 people who contracted infections at the surgery site, and one who died during the procedure. Because of the risks, the authors recommend that surgeons carefully select the people who are most likely to benefit from DBS.
Not everyone with Parkinson’s is a good candidate for DBS. Recipients must have had a PD diagnosis for at least five years to ensure they really have Parkinson’s. (Diagnostic accuracy for PD is low.) Surgeons generally restrict the treatment to people younger than 70, who they reason are best able to handle the risks of brain surgery and most likely to benefit from the chance to lead a more physically active life, said Elena Moro of Toronto Western Hospital in Ontario.
The people who respond best to surgery are those who already respond well to medication. Those people turn to DBS because the medication, after some years, often begins to cause uncomfortable side effects, such as the involuntary movements of dyskinesia, or because the medication wears off too quickly. For people who have taken PD meds such as levodopa for several years, life is a continuous cycle of “on” time, in which the drugs control symptoms well, and “off” time, when the medication wears off and they find themselves slow, trembling, even frozen in mid-movement. Peter and Barbara Higgs, both retired teachers in Workworth, Ontario, recall planning their outings and travels around the “on/off” cycle of Peter, who has Parkinson’s. Now “on” much of the time with his stimulator, Peter walked the 5K Parkinson SuperWalk in Peterborough, Ontario, last year. On average, people with DBS gain more than four hours of “on” time each day (see ARF related news story on Weaver et al., 2009).
However, people who do not find levodopa helpful may actually have a condition different from the “standard” Parkinson’s, such as dementia with Lewy bodies (DLB) or progressive supranuclear palsy, and the surgery is unlikely to help them. Some 15 percent of people with Parkinson’s meet the eligibility requirements for DBS, according to Moro.
Long follow-up studies show that even after five years—the maximum time yet published—DBS continues to support mobility. Among five people in a recent study of DBS recipients, subjects had nearly 60 percent less motor trouble while “off” medication than they did before the operation five years earlier. And the average dose of levodopa these people were taking dropped by half (Zabek et al., 2010). In another study of 49 people, researchers found similar improvements, although they also recorded some worsening of symptoms between the first and fifth year post-surgery (Krack et al., 2003). However, the authors noted that is to be expected from a progressive disease such as Parkinson’s.
Mysterious Ways
These outcomes are fairly impressive for a treatment that neither doctors nor scientists truly understand. DBS has its roots in chronic pain and tremor treatments of the 1970s, in which surgeons would simply destroy the area they thought was causing the problem. During the 1980s and 1990s, doctors found they could get similar effects by stimulating the area, a reversible treatment (Benabid et al., 1987; Benabid et al., 1991). “Maybe we excite cells with the high frequency, or maybe we change the pattern of discharge of these neurons, making them more normal,” Moro said. “Nobody really knows.”
Researchers also remain uncertain which brain region is the ideal target for Parkinson disease. In the majority of DBS operations, surgeons implant electrodes into the subthalamic nucleus (STN). This area is part of a circuit that connects dopamine production in the substantia nigra, commonly reduced in PD, to movement. Another part of this circuit is the globus pallidus internal segment (GPi), which also occasionally finds itself targeted by DBS. Each has its merits. The STN is easier to find in the brain and smaller, making it easier to place the electrode exactly where it belongs. But some scientists think that GPi stimulation minimizes side effects (Okun et al., 2009). Researchers are currently comparing the two regions directly in clinical studies.
The motor circuit that contains both the STN and GPi also feeds into non-motor functions. Therefore, DBS can have unintended consequences. Most side effects are mild; they include tingling in the limbs, slight paralysis, slurred speech, and loss of balance. They are often transient, occurring just after the stimulation turns on, or can be controlled by reducing the voltage delivered to the brain electrodes. Speech problems are of special concern, since some people with Parkinson’s already struggle to speak at a steady volume and enunciate words clearly. In a survey of 249 people with Parkinson’s, 99 of whom had had DBS, the nonprofit Parkinson Alliance found that speech dysfunction was a problem in both groups, but more so in the DBS subjects (Parkinson Alliance, 2009 [.pdf]).
Cognitive function and sleep are also areas of current interest, Moro said. In another Parkinson Alliance survey of 87 people with DBS and 76 without, the group found that people with DBS reported longer and less disturbed sleep (Parkinson Alliance, 2007 [.pdf]). As far as cognition is concerned, some DBS recipients show slightly reduced verbal fluency and vocabulary, working memory, and processing speed (Williams et al., 2010; Weaver et al., 2009). However, Moro said, these are decrements that come up on specific neuropsychological tests, and are unlikely to cause problems in a person’s day-to-day life.
“For a lot of people, it is just the simple everyday things that make the most difference,” Ryan said. Successful DBS could mean regaining the ability to shave, hold a glass full up to the brim, or toss a baseball to a child. Or even simply to express emotion, Peter Higgs said: “I can smile again.”—Amber Dance.
This is Part 1 of a four-part series. View a PDF of Part 1. See Part 2, Part 3, and Part 4. See PDF of entire series.
References
News Citations
- Deep-Brain Stimulation: Steadies the Body, But What About the Mind?
- Deep-Brain Stimulation: An Electrode for All Occasions?
- Deep-Brain Stimulation: There’s Still Room for Improvement
- PD Studies Highlight Deep Brain Stimulation, New Role for α-Synuclein
Paper Citations
- Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, Scott R, Ives N, Rick C, Daniels J, Patel S, Wheatley K, . Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 2010 Jun;9(6):581-91. PubMed.
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD, . Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009 Jan 7;301(1):63-73. PubMed.
- Zabek M, Sobstyl M, Koziara H, Kadziołka B, Mossakowski Z, Dzierzecki S. Bilateral subthalamic nucleus stimulation in the treatment of advanced Parkinson's disease. Five years' personal experience. Neurol Neurochir Pol. 2010 Jan-Feb;44(1):3-12. PubMed.
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003 Nov 13;349(20):1925-34. PubMed.
- Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50(1-6):344-6. PubMed.
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991 Feb 16;337(8738):403-6. PubMed.
- Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, Suelter M, Jacobson CE, Wang X, Gordon CW, Zeilman P, Romrell J, Martin P, Ward H, Rodriguez RL, Foote KD. Cognition and mood in Parkinson's disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009 May;65(5):586-95. PubMed.
Other Citations
External Citations
Further Reading
Papers
- Stefani A, Fedele E, Galati S, Raiteri M, Pepicelli O, Brusa L, Pierantozzi M, Peppe A, Pisani A, Gattoni G, Hainsworth AH, Bernardi G, Stanzione P, Mazzone P. Deep brain stimulation in Parkinson's disease patients: biochemical evidence. J Neural Transm Suppl. 2006;(70):401-8. PubMed.
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009 Apr 17;324(5925):354-9. PubMed.
- Freund HJ, Kuhn J, Lenartz D, Mai JK, Schnell T, Klosterkoetter J, Sturm V. Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol. 2009 Jun;66(6):781-5. PubMed.
- Okun MS, Foote KD. Enough is enough: moving on to deep brain stimulation in patients with fluctuating Parkinson disease. Arch Neurol. 2009 Jun;66(6):778-80. PubMed.
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J, . A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006 Aug 31;355(9):896-908. PubMed.
- Tuite PJ, Maxwell RE, Ikramuddin S, Kotz CM, Kotzd CM, Billington CJ, Billingtond CJ, Laseski MA, Thielen SD. Weight and body mass index in Parkinson's disease patients after deep brain stimulation surgery. Parkinsonism Relat Disord. 2005 Jun;11(4):247-52. PubMed.
Deep-Brain Stimulation: Steadies the Body, But What About the Mind?
With a few electrodes deftly threaded into a troubled brain, surgeons can often still the embarrassing tremors and alleviate the painful muscle cramps that come with Parkinson disease. But this deep-brain stimulation does not treat all that Parkinson’s does to a person. People may still have to cope with declining cognitive abilities, speech difficulties, or poor impulse control. And although DBS can quell some symptoms, it does not halt the inexorable progression of neurodegenerative disease. Sometimes, aspects of a person’s life—success at work, or in personal relationships—suffer following the huge upheaval that successful DBS surgery tends to bring with it. DBS can even break up a marriage.
Despite doctors’ best efforts, some people have unrealistic expectations for their outcomes, said Elena Moro of Toronto Western Hospital in Ontario. Unfortunately, the surgery’s results are not a sure thing, and people’s hopes are sometimes dashed. “It’s not working for me,” wrote J.R. Jay, a retired woman in Mobile, Alabama, in an e-mail to ARF. “I can’t tell you how I feel.” Jay had DBS for essential tremor. She has now been told the malfunctioning equipment necessitates a second surgery, but is seeking a second opinion. “I’m not happy,” she wrote.
Less Than a Miracle
People go through many changes following DBS, some positive, and some negative. In a survey [.pdf] conducted by the nonprofit Parkinson Alliance, people who had DBS reported less anxiety than those whose Parkinson’s was managed by medication alone. However, reports of depression were no different between the groups.
Some aspects of disease may remain the same, or even worsen, with deep-brain stimulation. For example, DBS can adversely affect speech, already a problem in people with PD—although in at least one case, DBS relieved stuttering in a person with PD (Walker et al., 2009). Impulsive behavior, such as hypersexuality or addiction to gambling, may remain after DBS or even worsen. In one instance, scientists in Amsterdam, The Netherlands, reported on a man who got hooked on slot machines after his DBS surgery. He had no history of compulsive gambling; indeed, family members described him as “stingy.” Dopamine agonists can also influence impulsive behavior, so his doctors were eventually able to control the compulsion by altering his medication (Smeding et al., 2007).
Emotional responses, too, may be altered by DBS. In a recent Danish study comprising in-depth interviews with people before and after DBS, one person told the researchers, “I have become much more sensitive; I cry over nothing” (Voon et al., 2008). Another research group found that following DBS, many people with Parkinson’s are less able to recognize the emotion in other people’s faces—particularly fear and sadness (Péron et al., 2010).
The potential side effects worried Greg Rice, a retired banker in Dover, Massachusetts, who had DBS for Parkinson’s five years ago. Rice has had Parkinson’s since 1993. Before his surgery, he had developed a late-blooming musical streak, composing symphonies. He suspected that his disease, plus a divorce in the late 1990s, unleashed his creativity, and with Parkinson’s keeping him from sleeping, he spent many night hours composing. He initially feared DBS would give him back mobility but take away this creativity. However, when he reached a point where he fell down 30 times a day, Rice decided the risk was worth taking.
Post-op, Rice is happy to still be composing, with the added benefit that he no longer falls down so often. Most importantly, he told ARF, the involuntary dyskinesia has stopped. “I would do it again even if I did lose the creativity, because the dyskinesia was driving me crazy,” Rice said. However, the surgery did not solve everything; he still struggles to speak intelligibly and sometimes gets “frozen,” crashing into things when he unfreezes. He copes with his disease by focusing on what he can do for others, working with his church, and coaching his daughter’s softball team. He was careful to note that DBS is not for everyone.
New Body, New Life
Symptomatic improvement in one arena does not always translate to the rest of a person’s life. For example, in a study of 23 people, researchers in Houston, Texas, found that people generally experienced greater energy levels and needed less help from a carer following DBS. But at the same time, the researchers found, some DBS recipients were unable or unwilling to apply their newfound abilities to succeed in the workplace, maintain interpersonal relationships, or to do more fun activities (Ferrara et al., 2010).
Researchers in France, who conducted pre- and post-op interviews with 29 DBS recipients, found similar results. In fact, they wrote, “Marital life and professional activity…worsened more often than they improved.” Of 16 participants who were working before surgery, only nine returned to work afterward. Social lives generally improved, but some participants reported that although they now had the physical ability to go out, they had no friends to see (Schüpbach et al., 2006).
The French study included 12 couples that had no notable marital problems before the surgery. In five of those couples, conflict arose post-operatively. It turned out that surgery changed a caregiver-patient dynamic that many couples were used to. In the study, the researchers noted one woman who spoke of her husband’s surgery: “When he was sick, we were a perfect couple. Now he wants to live the life of a young man, go out, meet new people; all of that is intolerable! I would rather he be like he was before, nice and docile!”
Families tend to stick together when someone is sick, said Helen Mayberg of Emory University in Atlanta, who was not involved in the study. But once a partner is physically independent, some caregivers may no longer feel obligated to stay in the relationship. Alternatively, people who previously needed care may want out of a relationship, and DBS gives them the opportunity to leave. Anthony Lang, a leading Parkinson’s physician-researcher at the Toronto Western Research Institute, noted in a plenary lecture at the 2009 AD/PD conference in Prague, Czech Republic, that response to DBS has become one of the more common reasons for divorce among PD patients. This has prompted some discussion among experts on whether the surgery should be offered at earlier stages of disease to people who are still more active and better able to make the most of their motor improvement without upending their pre-op life.
Any major surgery can cause upheaval, and for some people, the change is too much to bear. Researchers in a 55-center, global survey of more than 5,000 people who had DBS for Parkinson’s found the overall rate of successful suicide was 0.45 percent, and the attempt rate was 0.90 percent. When they compared post-DBS rates to the general population suicide rates in each country in the study, they found that DBS increased the suicide risk. The increased suicide rates continued for at least four years following the operation (Voon et al., 2008). Both researchers and patients suggest that a DBS team should include not only physicians and neurologists, but also psychologists or psychiatrists to guide people through the transition.
And in most cases, it is a transition worth making. For example, retired teachers Barbara and Peter Higgs, of Workworth, Ontario, were thrilled with the results of Peter’s DBS for Parkinson’s. “I am not that keen on being a caretaker, to tell you the truth,” Barbara Higgs said. “I was delighted to see him regain control of his life.” And the French study authors noted that despite mixed results from their surgery, none of the 29 people in their study wanted to turn the device off.—Amber Dance.
This is Part 2 of a four-part series. View a PDF of Part 2. See Part 1, Part 3, and Part 4. See PDF of entire series.
References
News Citations
- Deep-Brain Stimulation: Decade of Surgical Relief, Not Just for PD
- Deep-Brain Stimulation: An Electrode for All Occasions?
- Deep-Brain Stimulation: There’s Still Room for Improvement
Paper Citations
- Walker HC, Phillips DE, Boswell DB, Guthrie BL, Guthrie SL, Nicholas AP, Montgomery EB, Watts RL. Relief of acquired stuttering associated with Parkinson's disease by unilateral left subthalamic brain stimulation. J Speech Lang Hear Res. 2009 Dec;52(6):1652-7. PubMed.
- Smeding HM, Goudriaan AE, Foncke EM, Schuurman PR, Speelman JD, Schmand B. Pathological gambling after bilateral subthalamic nucleus stimulation in Parkinson disease. J Neurol Neurosurg Psychiatry. 2007 May;78(5):517-9. PubMed.
- Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schüpbach M, D'Ambrosia J, Thobois S, Tamma F, Herzog J, Speelman JD, Samanta J, Kubu C, Rossignol H, Poon YY, Saint-Cyr JA, Ardouin C, Moro E. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson's disease. Brain. 2008 Oct;131(Pt 10):2720-8. PubMed.
- Péron J, Biseul I, Leray E, Vicente S, Le Jeune F, Drapier S, Drapier D, Sauleau P, Haegelen C, Vérin M. Subthalamic nucleus stimulation affects fear and sadness recognition in Parkinson's disease. Neuropsychology. 2010 Jan;24(1):1-8. PubMed.
- Ferrara J, Diamond A, Hunter C, Davidson A, Almaguer M, Jankovic J. Impact of STN-DBS on life and health satisfaction in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010 Mar;81(3):315-9. PubMed.
- Schüpbach M, Gargiulo M, Welter ML, Mallet L, Béhar C, Houeto JL, Maltête D, Mesnage V, Agid Y. Neurosurgery in Parkinson disease: a distressed mind in a repaired body?. Neurology. 2006 Jun 27;66(12):1811-6. PubMed.
Other Citations
External Citations
Further Reading
Papers
- Le Jeune F, Drapier D, Bourguignon A, Péron J, Mesbah H, Drapier S, Sauleau P, Haegelen C, Travers D, Garin E, Malbert CH, Millet B, Vérin M. Subthalamic nucleus stimulation in Parkinson disease induces apathy: a PET study. Neurology. 2009 Nov 24;73(21):1746-51. PubMed.
- Antonini A, Cilia R. Behavioural adverse effects of dopaminergic treatments in Parkinson's disease: incidence, neurobiological basis, management and prevention. Drug Saf. 2009;32(6):475-88. PubMed.
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J, . A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006 Aug 31;355(9):896-908. PubMed.
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD, . Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009 Jan 7;301(1):63-73. PubMed.
- Dowding CH, Shenton CL, Salek SS. A review of the health-related quality of life and economic impact of Parkinson's disease. Drugs Aging. 2006;23(9):693-721. PubMed.
- Zand R. Is dopamine agonist therapy associated with developing pathological gambling in Parkinson's disease patients?. Eur Neurol. 2008;59(3-4):183-6. PubMed.
Deep-Brain Stimulation: An Electrode for All Occasions?
Deep-brain stimulation (DBS) is not just for shakes and trembles any more. With the advent of improved brain imaging, researchers are linking certain parts of brain anatomy to conditions ranging from addiction to Alzheimer disease. And for every MRI or CT scan showing a hot spot, it seems, there increasingly is a surgeon waiting to stick an electrode in it. “I do not think there is any disease that is ‘safe’ right now,” quipped Jerrold Vitek, a dementia with Lewy bodies (DLB) pioneer who is set to take over the Department of Neurology at the University of Minnesota in Minneapolis-St. Paul this July. “If you have a hammer, everything looks like a nail.”
DBS is creeping into the realm of psychiatric disorders such as obsessive-compulsive disorder (OCD), Tourette syndrome, and depression. Small studies show that the treatment is often effective and causes minimal side effects (reviewed in Kuhn et al., 2010). In part, the use of DBS in psychiatric conditions was inspired by unexpected results when doctors attempted stimulation to treat Parkinson’s. A couple of people with both PD and OCD, who received DBS for the Parkinson’s symptoms, also experienced a reduction in obsessive and compulsive behaviors (Mallet et al., 2002). In another case, a woman who received DBS to relieve Parkinson’s experienced profound sadness when certain parts of her brain were stimulated. The effect quickly disappeared when the electrodes were turned off (Bejjani et al., 1999). These side effects led researchers to suspect that thoughts and feelings, as well as movement, could be subject to alteration by DBS. Conditions such as Tourette’s (reviewed in Temel and Visser-Vandewalle, 2004) and OCD (Jung et al., 2006) have also responded to deep brain lesions, a precursor to DBS used in the past.
Diminishing Depression
Given the small but real risks inherent in brain surgery, DBS tends to become an option when a disease is serious and other therapies have failed. There are many conditions that, at least in some people, resist the best medicine currently on offer. For example, Helen Mayberg of Emory University in Atlanta, Georgia, works on treatment-resistant depression (TRD). The patients she works with have had major depression for years, and tried medication, psychological therapy, and electroconvulsive therapy, to no avail. Many are unable to work. “They are dangerously and intractably ill,” she said.
Mayberg was able to turn the images she saw on brain scans of sad or depressed people into a novel therapy for the condition. This is the first time, Mayberg said, that a targeted treatment came directly from images, with no other basis for potential efficacy. She and others observed that the subgenual cingulate cortex is overactive in people with TRD. Activity in the region also turns up with negative mood in healthy people (Mayberg et al., 1999). “We are interested in turning the activity down,” Mayberg said.
Mayberg, then at the University of Toronto in Ontario, initially tried DBS of the subgenual cingulate in six people (Mayberg et al., 2005). Later, she and others expanded the trial to include 14 more patients (Lozano et al., 2008). They saw effects even in the operating room, when surgeons stimulated the target area to make sure electrodes were properly placed. Spontaneously, some subjects reported that the room suddenly looked brighter, or that they experienced a “disappearance of the void” (Mayberg et al., 2005). By six months after the surgery, 60 percent of recipients had some response, and 35 percent were considered in remission (Lozano et al., 2008). DBS for depression is currently under trial in a study run by St. Jude Medical, Inc., of St. Paul, Minnesota, a maker of DBS devices, Mayberg said.
Mayberg cautioned that DBS marks only the beginning of a person’s recovery from TRD. “This does not make you happy,” she said. “This turns negative off.” The recipient must take the next step to regain a positive outlook. And just as a person who has, say, hip replacement surgery needs rehab, people who have DBS for depression need “psychological rehab,” Mayberg said, to help them adjust.
If I Had a Hammer….
Currently, DBS is FDA-approved for essential tremor, Parkinson disease, and dystonia. The FDA also allows some people with OCD to receive the device. Efficacy for this condition is under study but remains unproven. Beyond that, researchers are trying DBS for a whole laundry list of conditions (reviewed in Awan et al., 2009). Clinical trials are underway for Huntington disease, cluster headache, pain, epilepsy, and Tourette syndrome.
Some scientists hope that even conditions that cause their primary pathology outside of the brain may respond to DBS. For example, amyotrophic lateral sclerosis manifests primarily in the spinal cord—but researchers using single-photon emission computed tomography discovered lesions in the cortex of four people with ALS. They attempted DBS with these four in a preliminary study. Two years later, two of the people had only mild progression of the illness, which is normally fatal within three to five years, and their lesions had disappeared. The third recipient’s disease continued to progress after the first few months; the fourth, however, committed suicide (Sidoti and Agrillo, 2006).
Most DBS targets are based on what researchers know about how the brain works, but at least one, for Alzheimer disease, was discovered by more roundabout means. In 2008, researchers from Toronto Western Hospital in Ontario reported on a surprising finding (Hamani et al., 2008). They were hoping to help an obese man stem his desire for food. The 50-year-old, 420-pound man had tried dieting, psychological therapy, and medication without success. He feared that even if he received bariatric surgery, he would continue to overeat.
The researchers targeted the hypothalamus, an area known to influence feeding in animals (Takaki et al., 1992). In the past, doctors had targeted this region with lesions to treat obesity (Quaade, 1974).
During the surgery, the doctors turned on the signal to the electrodes to ensure they were hitting the target area. They asked the man, who was awake during the procedure, if he felt any change in hunger. He did not—but he did notice a strange sense of déjà vu. Suddenly, he flashed back 30 years to a scene in a park, surrounded by friends. He recognized his girlfriend from that time. When the doctors turned off the stimulation, the memory vanished; they turned it back on and the memory resurfaced.
“We were caught completely by surprise,” said study author Andres Lozano. “We knew immediately that this was something very significant.”
The hypothalamus is involved in memory as well as appetite (Soriano-Mas et al., 2005). In tests following the surgery, the researchers also found the obese man was more likely to remember word pairings when the stimulator was on; using electromagnetic tomography, they saw that the stimulation activated the brain’s memory circuit. (They used this method because the man was too large for a standard PET or MRI scan.) As to the effect on obesity, the man reported reduced cravings and did lose more than 25 pounds, but gained them back when he started turning off the neurostimulator to snack at night.
Based on this serendipitous, if anecdotal, finding, the researchers launched a small trial with six subjects to see if DBS can improve memory in six people with mild Alzheimer disease. Although DBS cannot repair the degenerated tissue lost in Alzheimer’s, Lozano hopes that it can improve input to “innocent bystander” parts of the brain that, while healthy themselves, are missing input from damaged neurons. Lozano expects to publish the results within the next few months. Researchers in the French city of Nice in 2009 listed a similar trial, though it is not recruiting yet.
In a related study, researchers in Germany this year began testing DBS for Alzheimer’s. Unlike the Toronto and the Nice groups, who are targeting the fornix region, this group is tickling the nucleus basalis of Meynert, an area known to degenerate in AD (see ARF related news story on Freund et al., 2009).
“These are exciting times,” Mayberg said of the rapidly expanding DBS field. “As we learn more about the brain, we are going to be able to help people in ways we had not thought about before.”—Amber Dance.
This is Part 3 of a four-part series. View a PDF of Part 3. See Part 1, Part 2, Part 4. See PDF of entire series.
References
News Citations
- Meynert, Oh, My! Deep Brain Stimulation to Treat Dementia?
- Deep-Brain Stimulation: Decade of Surgical Relief, Not Just for PD
- Deep-Brain Stimulation: Steadies the Body, But What About the Mind?
- Deep-Brain Stimulation: There’s Still Room for Improvement
Paper Citations
- Kuhn J, Gründler TO, Lenartz D, Sturm V, Klosterkötter J, Huff W. Deep brain stimulation for psychiatric disorders. Dtsch Arztebl Int. 2010 Feb;107(7):105-13. PubMed.
- Mallet L, Mesnage V, Houeto JL, Pelissolo A, Yelnik J, Behar C, Gargiulo M, Welter ML, Bonnet AM, Pillon B, Cornu P, Dormont D, Pidoux B, Allilaire JF, Agid Y. Compulsions, Parkinson's disease, and stimulation. Lancet. 2002 Oct 26;360(9342):1302-4. PubMed.
- Bejjani BP, Damier P, Arnulf I, Thivard L, Bonnet AM, Dormont D, Cornu P, Pidoux B, Samson Y, Agid Y. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999 May 13;340(19):1476-80. PubMed.
- Temel Y, Visser-Vandewalle V. Surgery in Tourette syndrome. Mov Disord. 2004 Jan;19(1):3-14. PubMed.
- Jung HH, Kim CH, Chang JH, Park YG, Chung SS, Chang JW. Bilateral anterior cingulotomy for refractory obsessive-compulsive disorder: Long-term follow-up results. Stereotact Funct Neurosurg. 2006;84(4):184-9. PubMed.
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999 May;156(5):675-82. PubMed.
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005 Mar 3;45(5):651-60. PubMed.
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008 Sep 15;64(6):461-7. PubMed.
- Awan NR, Lozano A, Hamani C. Deep brain stimulation: current and future perspectives. Neurosurg Focus. 2009 Jul;27(1):E2. PubMed.
- Sidoti C, Agrillo U. Chronic cortical stimulation for amyotropic lateral sclerosis: a report of four consecutive operated cases after a 2-year follow-up: technical case report. Neurosurgery. 2006 Feb;58(2):E384; discussion E384. PubMed.
- Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, Wennberg RA, Lozano AM. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008 Jan;63(1):119-23. PubMed.
- Takaki A, Aou S, Oomura Y, Okada E, Hori T. Feeding suppression elicited by electrical and chemical stimulations of monkey hypothalamus. Am J Physiol. 1992 Apr;262(4 Pt 2):R586-94. PubMed.
- Quaade F. Letter: Stereotaxy for obesity. Lancet. 1974 Feb 16;1(7851):267. PubMed.
- Soriano-Mas C, Redolar-Ripoll D, Aldavert-Vera L, Morgado-Bernal I, Segura-Torres P. Post-training intracranial self-stimulation facilitates a hippocampus-dependent task. Behav Brain Res. 2005 May 7;160(1):141-7. PubMed.
- Freund HJ, Kuhn J, Lenartz D, Mai JK, Schnell T, Klosterkoetter J, Sturm V. Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol. 2009 Jun;66(6):781-5. PubMed.
Other Citations
External Citations
Further Reading
Papers
- Bonelli RM, Gruber A. Deep brain stimulation in Huntington's disease. Mov Disord. 2002 Mar;17(2):429-30; author reply 431-2. PubMed.
- Timmermann L, Pauls KA, Wieland K, Jech R, Kurlemann G, Sharma N, Gill SS, Haenggeli CA, Hayflick SJ, Hogarth P, Leenders KL, Limousin P, Malanga CJ, Moro E, Ostrem JL, Revilla FJ, Santens P, Schnitzler A, Tisch S, Valldeoriola F, Vesper J, Volkmann J, Woitalla D, Peker S. Dystonia in neurodegeneration with brain iron accumulation: outcome of bilateral pallidal stimulation. Brain. 2010 Mar;133(Pt 3):701-12. PubMed.
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD, . Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009 Jan 7;301(1):63-73. PubMed.
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J, . A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006 Aug 31;355(9):896-908. PubMed.
- Shields DC, Sharma N, Gale JT, Eskandar EN. Pallidal stimulation for dystonia in pantothenate kinase-associated neurodegeneration. Pediatr Neurol. 2007 Dec;37(6):442-5. PubMed.
- van Balken I, Litvan I. Current and future treatments in progressive supranuclear palsy. Curr Treat Options Neurol. 2006 May;8(3):211-23. PubMed.
News
- Deep-Brain Stimulation: Decade of Surgical Relief, Not Just for PD
- Deep-Brain Stimulation: Steadies the Body, But What About the Mind?
- Mind-machine Meld: Brain-computer Interfaces for ALS, Paralysis
- PD Studies Highlight Deep Brain Stimulation, New Role for α-Synuclein
- Parkinson Therapies Go Deep and Shallow
- Meynert, Oh, My! Deep Brain Stimulation to Treat Dementia?
Deep-Brain Stimulation: There’s Still Room for Improvement
Though some 55,000 people have received deep-brain stimulation for conditions ranging from Parkinson disease to obsessive-compulsive disorder, there is still plenty of room to improve the process. Companies are working on smaller devices with longer battery life. Surgeons are seeking ways to more safely and precisely implant the electrodes that reset faulty brain signals. And people who receive DBS are hoping that researchers can improve the sometimes lengthy and cumbersome process of fine-tuning the settings on the pacemaker-like neurostimulator that controls the pulses to the electrodes.
Ever-improving imaging technology has been key to the spread of DBS, as doctors identify more brain regions that might benefit from the stimulation. For example, researchers have gone as high as seven-Tesla MR imaging, in comparison to the standard two Tesla scanners more routinely available (Cho et al., 2010). These high-resolution images help surgeons target the right spot. “Getting the electrodes in the right place is probably the most important thing to dictate clinical outcomes,” said Cameron McIntyre of the Cleveland Clinic. A millimeter to the left or right, and results will be less impressive than they could be. And this placement happens some six centimeters below the skull, where surgeons cannot navigate by sight.
In the OR
To help surgeons turn the images from an MRI or CT scan into a plan of operation, McIntyre and others have developed a software package called Cicerone (Miocinovic et al., 2007). The software lines up a person’s brain scans with standard neurophysiology maps and 3D brain atlases, so surgeons can identify the most promising site for stimulation and plan a route to get there. During the operation itself, the surgeon can also use the images to visualize the electrode’s location, and Cicerone will predict what regions it will stimulate.
In addition to placing the electrode, better images help doctors plan a safe surgery. The location of blood vessels varies from person to person, and surgeons may nick one, causing a hemorrhage. The risk of serious bleeding, leading to brain damage or death, is between 1 and 2 percent. Neurologist Jerrold Vitek, soon to move from Ohio’s Cleveland Clinic to the University of Minnesota in Minneapolis-St. Paul, said he wants to see that risk drop to 0.5 percent, so more people who might benefit from DBS will feel confident signing up for the procedure.
A person who receives DBS will likely need brain surgery once, but will also have a second surgery to implant the neurostimulator in the chest. It connects to the brain via wires under the neck’s skin. That surgery is necessary every time the neurostimulator’s batteries run out and require replacement—once every two to five years, depending on the voltage settings.
But Richard McEnery, a software developer and photographer in Sammamish, Washington, was burning through batteries in a year, he told ARF. DBS silenced much of his dystonia—a condition that includes involuntary muscle contraction—including the neck tremors that made him “look like a bobblehead doll,” he said. Now, McEnery benefits from a relatively new improvement in DBS technology, a rechargeable battery. It doesn’t last as long—perhaps a month—but he can top it off at home while watching TV. The recharger is a large plastic device connected to a battery. Once a week, McEnery dons a harness that aligns the recharger with the neurostimulator under his skin. Over an hour or two, the device wirelessly transmits power through his skin to the neurostimulator, and he is powered up and good to go.
Post-Op
DBS is not plug-and-play; the equipment requires setting and maintenance. Neurostimulator settings include many parameters; voltage, signal length, and frequency of stimulation are just a few. A doctor or nurse works with the DBS recipient to program the neurostimulator’s activity. Altogether, thousands of possible setting combinations exist, and every recipient needs a personalized one. Finding those magic numbers requires multiple office visits that can last hours. The time involved—and lack of many healthcare practitioners skilled in setting the device—is one of the biggest complaints among people who have DBS. McEnery recalls it took a year to perfect his settings.
The programming task frequently falls to nurses, neurophysiologists, and doctors still in training, who may or may not be expert in the process and in medical treatments for the condition at hand. In a 2006 study, researchers at Toronto Western Hospital examined the potential for an experienced neurologist to improve programming (Moro et al., 2006). They initiated the study when Elena Moro, an expert in DBS as well as management of Parkinson’s and movement disorders, joined the clinic. She reset neurostimulators for 44 people with Parkinson’s who had had DBS for an average of 3.5 years already. The result: more than half of the participants saw improvement in mobility and daily activities, and were able to reduce their anti-Parkinson’s medications. Others saw no benefit; in four people, symptoms worsened. The data suggest, the authors write, that the expertise of the programmer makes a difference.
“A great deal of clinical intuition goes into the process,” said McIntyre, who was not involved with the Toronto study. Yet again, he offers a computational solution. He and colleagues are developing StimExplorer, a program that integrates a patient’s MR images with the position of the electrode (Butson et al., 2007). It offers users a set of theoretically optimal parameter settings. They may not be just right, but they should, hopefully, put the user in the right ballpark from which fine-tuning is easier. McIntyre has licensed much of his technology for commercial development, and hopes his software will receive FDA approval in a year or two. Something like StimExplorer could help inexperienced programmers, Moro said.
As DBS becomes more popular, more companies are developing stimulators. Medtronic, Inc., headquartered in Minneapolis, Minnesota, currently holds much of the U.S. market. St. Jude Medical, Inc., based in St. Paul in the same state, already has a DBS device approved for Parkinson’s in Europe and is currently conducting a U.S. study. Competition should lead to improvements in the technology. For example, doctors hope to soon have a neurostimulator small enough to fit in the head, against the skull. This would eliminate the wires traveling through the neck, which might break. “I think you will see a lot of one-upmanship between the two players,” McIntyre said.—Amber Dance.
This is Part 4 a four-part series. View a PDF of Part 4. See Part 1, Part 2, and Part 3. See PDF of entire series.
Comments
No Available Comments
Make a Comment
References
News Citations
- Deep-Brain Stimulation: Decade of Surgical Relief, Not Just for PD
- Deep-Brain Stimulation: Steadies the Body, But What About the Mind?
- Deep-Brain Stimulation: An Electrode for All Occasions?
Paper Citations
- Cho ZH, Min HK, Oh SH, Han JY, Park CW, Chi JG, Kim YB, Paek SH, Lozano AM, Lee KH. Direct visualization of deep brain stimulation targets in Parkinson disease with the use of 7-tesla magnetic resonance imaging. J Neurosurg. 2010 Sep;113(3):639-47. PubMed.
- Miocinovic S, Noecker AM, Maks CB, Butson CR, McIntyre CC. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochir Suppl. 2007;97(Pt 2):561-7. PubMed.
- Moro E, Poon YY, Lozano AM, Saint-Cyr JA, Lang AE. Subthalamic nucleus stimulation: improvements in outcome with reprogramming. Arch Neurol. 2006 Sep;63(9):1266-72. PubMed.
- Butson CR, Noecker AM, Maks CB, McIntyre CC. StimExplorer: deep brain stimulation parameter selection software system. Acta Neurochir Suppl. 2007;97(Pt 2):569-74. PubMed.
Other Citations
Further Reading
Papers
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD, . Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009 Jan 7;301(1):63-73. PubMed.
- Hertel F, Züchner M, Weimar I, Gemmar P, Noll B, Bettag M, Decker C. Implantation of electrodes for deep brain stimulation of the subthalamic nucleus in advanced Parkinson's disease with the aid of intraoperative microrecording under general anesthesia. Neurosurgery. 2006 Nov;59(5):E1138; discussion E1138. PubMed.
- Amirnovin R, Williams ZM, Cosgrove GR, Eskandar EN. Experience with microelectrode guided subthalamic nucleus deep brain stimulation. Neurosurgery. 2006 Feb;58(1 Suppl):ONS96-102; discussion ONS96-102. PubMed.
- Antonini A, Landi A, Benti R, Mariani C, De Notaris R, Marotta G, Pezzoli G, Gaini SM, Gerundini P. Functional neuroimaging (PET and SPECT) in the selection and assessment of patients with Parkinson's disease undergoing deep brain stimulation. J Neurosurg Sci. 2003 Mar;47(1):40-6. PubMed.
- Starr PA, Christine CW, Theodosopoulos PV, Lindsey N, Byrd D, Mosley A, Marks WJ. Implantation of deep brain stimulators into the subthalamic nucleus: technical approach and magnetic resonance imaging-verified lead locations. J Neurosurg. 2002 Aug;97(2):370-87. PubMed.
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J, . A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006 Aug 31;355(9):896-908. PubMed.
News
- Deep-Brain Stimulation: Decade of Surgical Relief, Not Just for PD
- Mind-machine Meld: Brain-computer Interfaces for ALS, Paralysis
- Flashy Technique Uses Light to Command Neural Firing
- Deep-Brain Stimulation: Steadies the Body, But What About the Mind?
- Deep-Brain Stimulation: An Electrode for All Occasions?
- PD Studies Highlight Deep Brain Stimulation, New Role for α-Synuclein
DBS Update: Attempting to Stimulate Memory in Alzheimer’s
Researchers are sliding electrodes into the brains of people with Alzheimer disease, hoping to awaken memories and stave off disease-induced forgetfulness. In the August 4 Annals of Neurology online, a team led by Andres Lozano at Toronto Western Hospital report on a small deep-brain stimulation (DBS) trial. They write that the therapy was safe and was able to alter brain metabolism.
DBS can quell the symptoms of Parkinson disease and is under investigation for a variety of conditions, including Huntington disease, pain, epilepsy, and Tourette syndrome (see ARF four-part news story on advances in this treatment technique). Lozano and colleagues first discovered its effect on memory when they stimulated the hypothalamus of an obese man. They were hoping to curb his appetite, but instead induced a flashback to a 30-year-old memory (Hamani et al., 2008).
Now, Lozano, first author Adrian Laxton, and colleagues have completed a Phase 1 trial on six participants with early Alzheimer’s. The physicians were successful in their primary goal—to show the treatment is safe—as none of the six suffered serious adverse effects.
Further, the scientists looked for evidence that the stimulation was increasing brain activity. Alzheimer disease dampens glucose metabolism in the temporal and parietal regions, so Laxton and the other researchers used positron emission tomography (PET) to examine metabolism after DBS. Changes occurred within one month of starting stimulation, with metabolism going up in both the temporal and parietal areas.
The researchers also examined the subjects for evidence of altered cognition. Standard measures such as the Mini-Mental State Examination (MMSE) and the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-cog) do not decrease linearly or regularly, so it is difficult to speculate how much decline the participants would have experienced without DBS. With no placebo control participants for comparison, the study was not able to clearly demonstrate cognitive effects. The researchers suggest that a few participants seemed to benefit, though, in that their scores improved or declined more slowly than might be expected.
Lozano and colleagues, satisfied with their results, are now planning a multicenter Phase 2 trial. The technique is currently approved by the FDA for Parkinson disease, essential tremor, and dystonia.—Amber Dance
Comments
-
It is quite interesting that stimulation of the fornix/hypothalamus influences memory, with notable effects on FDG-PET activity in the regions of abnormality in AD. With the success of DBS for Parkinson's, this approach is clearly feasible. Of course, much more work is required to determine whether DBS can produce clinically meaningful benefits in AD, mild cognitive impairment, or other amnestic disorders. But Andres Lozano and his colleagues should be applauded for this novel and intriguing approach.
View all comments by Paul Aisen -
I have studied Alzheimer disease (AD) since 1978, and since about 1980, I have seen some new ideas for treating AD nearly every month. Of these, five have been FDA approved and have modest benefits for patients (some improvement in cognition and behavior, and changing the course of the disease by a few months). I am sure that 300 good ideas have failed in this time, and there is nothing on the horizon that looks like it will stop AD.
This study of brain stimulation does not distinguish itself from other failed approaches at this point. The rationale for how this treatment would stop the development of senile plaques and neurofibrillary tangles is missing. The idea of stimulating the "default mode network" is also weak. The idea of stimulating the fornix is admirable, but I think it is a little too much wishful thinking to really conceive that this would help to stop Alzheimer pathology or improve memory in a dementia patient. If this approach could be shown to diminish amyloid deposition in the brain (even in a few locations), lower CSF tau, and show long-term improvement or even maintenance of cognitive and behavioral function, then it could be a tremendous advance.
There is a problem with the results of this study. The presented results do not show any clear benefit, and there is little attempt to correct the effects of the stimulation on the ADAS and MMSE for severity and age. These are important factors that need to be addressed. Evidence of beneficial effect on relevant biomarkers is becoming more important for accepting therapeutic interventions, and long-term demonstrations of clinical improvement, with careful control for specific ApoE genotypes, are also needed.
In summary, I think it was good that this study was done, but it remains to be seen whether it will have promise for the future.
View all comments by John (Wes) Ashford
Make a Comment
References
News Citations
Paper Citations
- Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, Wennberg RA, Lozano AM. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008 Jan;63(1):119-23. PubMed.
Further Reading
Papers
- Soriano-Mas C, Redolar-Ripoll D, Aldavert-Vera L, Morgado-Bernal I, Segura-Torres P. Post-training intracranial self-stimulation facilitates a hippocampus-dependent task. Behav Brain Res. 2005 May 7;160(1):141-7. PubMed.
- Freund HJ, Kuhn J, Lenartz D, Mai JK, Schnell T, Klosterkoetter J, Sturm V. Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol. 2009 Jun;66(6):781-5. PubMed.
- Aybek S, Lazeyras F, Gronchi-Perrin A, Burkhard PR, Villemure JG, Vingerhoets FJ. Hippocampal atrophy predicts conversion to dementia after STN-DBS in Parkinson's disease. Parkinsonism Relat Disord. 2009 Aug;15(7):521-4. PubMed.
A Day in the OR: Surgeons Zap Neurons for Parkinson’s, AD
The doctors crowd around the computer monitor, examining the brain scans of the man lying on their operating table down the hall. The metal headdress they have mounted to his cranium—or “stereotactic frame” in medical lingo—provides coordinates, a sort of 3-D GPS they will use to guide platinum-iridium electrodes deep into his brain. The electricity pulsing through those electrodes will temporarily short out his misfiring globus pallidus. Hopefully, that will provide relief from the slow, abnormal movements caused not only by Parkinson’s disease, but also by the medications he has taken to control it for the past eight years. Deep-brain stimulation (DBS) has become a standard therapy for Parkinson’s and essential tremor (see ARF related news story); it is being tested now in a Phase 2 multicenter trial for AD (see below).
The 66-year-old man in the University of California, Los Angeles (UCLA) operating room is Daniel Duran of Rolling Hills Estates, California. Duran has had PD since 2004. Like many people with his condition, he noticed that medications no longer work well. He freezes up during long “off” periods between doses, and his “on” periods after a dose last for only 90 minutes. Having to be near a chair as every on period ends makes it hard for Duran, a retired engineer, to keep up his schedule of walks, workouts, support group meetings, and lunch dates. His state emulates that of Cinderella, recalled his wife Brenda Duran after the procedure—except “it is turning midnight every hour and a half.” Daniel has also been suffering side effects, including hallucinations that wake him, terrified, in the middle of the night. With today’s surgical procedure, Antonio De Salles, who heads UCLA’s DBS program, aims to improve Duran’s movement and reduce his medications.
Brenda and Daniel Duran hoped DBS surgery would stifle the symptoms of Parkinson’s and side effects from too much medication. Image courtesy of Daniel Duran
Before DBS was available, De Salles performed some 300 pallidotomies, destroying forever the area responsible for the poor movement control. DBS is preferable, he said, because it is reversible and the stimulation can be varied to find the level with the best effects. The voltage De Salles will shoot through the electrodes during the operation will shut off the globus pallidus much like a lesion would. “I am overwhelming the system with electrical stimulation,” De Salles said. The depolarized neurons should take a break, and Duran will see his muscle rigidity diminish within just a few minutes, right here in the operating room (OR). However, when the doctors turn off the voltage, the neurons can fire again, so unwanted side effects are not permanent, as they are with pallidotomies.
Duran’s day started at 3:30 a.m., when he and Brenda rose to go to the hospital. He was not allowed to eat, drink, or take his Parkinson’s meds before the surgery, so he arrived parched and stiff. He tried to convince every nurse who stopped by his room to give him his meds, because he is so uncomfortable without them. “He is like a drug addict,” Brenda joked. Daniel told her, “You don’t know what it’s like … it makes it a long day.” He has been in the operating room since about 7:30, under light anesthesia as the doctors formulate their plan.
Mapmaking
Before they cut, the surgical team must decide exactly where they want to place the electrodes, and what route they will take to reach the target tissue. They need to avoid blood vessels and pockets of cerebrospinal fluid (CSF). Since the larger vessels tend to nestle in the grooves of the brain’s cortex, the surgeons will enter the delicate organ away from those spaces. “You do not want to go through valleys; you want to go through mountains,” said Ausaf Bari, a neurosurgery resident on the case. “You can go through a lot of brain without hurting anything.”
And, of course, the physicians will forbear inserting the wires in Duran’s forehead, because that would not look handsome. For this kind of surgery, the insertion sites typically sit atop the head, one on each side—about where antennae on a Halloween headband might protrude.
Today, De Salles has selected the internal globus pallidus (GPi) as the place to stimulate. The other option for Parkinson’s is the subthalamic nucleus (STN). Treatment in that area can be very effective, De Salles said; recipients typically cut their medication use by two-thirds. Targeting the GPi instead will probably mean that Duran can reduce his prescriptions by half, De Salles predicts, but it is a safer site for cognition. Older people with STN electrodes may become confused or aggressive. Thus, De Salles typically prefers the GPi for people who have passed 65. The GPi is also less risky for surgery, since it is larger and lies closer to the brain’s surface than does the STN (see ARF related news story on Follett et al., 2010, and ARF related news story on Weaver et al., 2012).
The frontal brain section illustrates DBS targets, including the subthalamic nucleus (STN) and internal globus pallidus (GPi). Image courtesy of Obeso et al., 2002. Reprinted with permission from The American Physiological Society.
At the computer, De Salles adjusts the plan to his liking, and Bari writes down the coordinates. He reads them back to make sure he made no mistake. “Let’s go do it, then,” De Salles says.
Go Time
Back in the OR—a large, white room with fluorescent lights and medical machinery—Michael Jackson’s “Smooth Criminal” plays quietly. Three monitors display Duran’s vitals. The doctors and nurses are careful not to bump against a blue-shrouded table holding their sterile instruments.
The surgeons, wearing blue scrubs and purple gloves, prepare for the operation. Five people adjust Duran’s head into just the right position for the surgery. Then, the anesthesiologist checks that he is still breathing easily. Bari shampoos his hair to minimize the risk of infection, since they are not shaving his entire scalp. Tufts of hair drop to the floor as they shave two spots. Bari draws the incision sites with a marker.
Next, the team adjusts a semicircular x-ray arm over Duran’s head. On each side of his head, they set up clear circles with crosshairs that will act as bullseyes for the electrodes. When the surgeons check their work, the electrode tips should appear right in the crosshairs on an x-ray image. Placing the x-ray targets takes time, but it is important. “Sometimes the little details make a big difference,” said Jacob Chodakiewitz, another surgeon on the team. The team hangs a plastic sheet from the x-ray arm, leaving just the top of Duran’s head exposed to the surgeons.
From the computed tomography scans taken earlier today, the doctors have determined the exact angle by which they will enter the brain with a guide tube holding the electrode. To find that angle, they use a large metal protractor mounted to the stereotactic frame on the man’s head.
Stereotactic frame as used in surgery to place deep-brain stimulation electrodes. Image courtesy of Elekta
The surgical nurse notes the time on a whiteboard as the doctors make the first cut. It is 10:24 a.m. They start on the left side of the head, making a small incision so they can see the bone underneath. The scalp tends to bleed a lot, so the surgeons quickly cauterize the tissue. They place a spreader to hold open the skin while the scrub nurse prepares the drill. It is smaller than a household drill, but the bit is long. The sound of the drill biting into the skull is surprisingly soft as Journey’s “Send Her My Love” plays in the background. Imagine a softer whine than the drill at your dentist’s office.
After all the planning and set up, inserting the electrode takes only a few minutes. The wire is live as the surgeons slide it in, and clinical specialist Eric Behnke calls out numbers as the electrode descends, “620 … 550 ….” He is measuring the resistance the tissue provides against the current, which tells the doctors where they are in the brain. CSF provides very little resistance; white matter, much more. By 10:30, it is time to wake up Duran and test the stimulation. The anesthesiologists turn off the propofol drip that has been keeping him sedated.
Open Your Eyes!
“Wake up!” Chodakiewitz yells—Duran is hard of hearing. “Open your eyes!” Duran moves his legs and picks at his blanket. Finally he mumbles, “I’m awake, I’m awake.”
The team will be looking for effects on the rigidity in Duran’s right hand, since they have operated on the left side of the brain. The main concern at this point is that the stimulation does not affect his thinking or cause other side effects. As Behnke ramps up the voltage—typical stimulation is 2 to 5 volts—Chodakiewitz asks Duran to count to 50 and sing, “Tie a Yellow Ribbon Round the Ole Oak Tree.” Duran succeeds in counting and warbling the chorus.
By 11:00, the doctors are satisfied that the stimulation is producing the desired effect without danger to other functions. The neuroanesthesiologist turns up the propofol again as the surgeons close the left incision and start on the right.
The second time Duran awakes, at 11:23, he knows the drill and offers up his right hand. Chodakiewitz gently takes the left instead for this round. Chodakiewitz notices that, as the voltage gets too high, Duran’s facial muscles contract. The GPi lies close to pathways that control the face, De Salles told Alzforum. Thus, seeing this side effect helped the doctors confirm the electrode was in the proper position (Gorgulho et al., 2009).
Once the team is satisfied with the second electrode’s stimulation, they let Duran drift back into the twilight of propofol sleep as they check the electrodes’ placement again on the x-ray and close the incision. They phone his wife and daughter to tell them the surgery went well. Later in the afternoon, around 4:00, Duran wakes up in the recovery room and demands his medication, telling nurses the drug names and precise dosages. At that point, Brenda Duran recalled later, she knew he must be okay.
The View From the Other Side of the Curtain
What is a DBS procedure like from the patient’s perspective? For his part, Duran remembers little from the OR, but Richard Thompson, a cartoonist in Arlington, Virginia, shared his story with Alzforum via e-mail, and with his fans via his blog. Thompson, who is 55, drew the syndicated comic strip Cul de Sac for five years; the final strip ran in September of 2012. Three years after his Parkinson’s diagnosis, he scheduled the operation for 12 October 2012. “It seemed like the next step that I should take in treatment, inevitable, so why put it off?” he wrote.
Without his medication, Thompson was wobbly when he got up at 4:00 a.m. to go to MedStar Georgetown University Hospital in Washington, DC. His wife and a friend helped him into the car. The empty streets on the chilly, foggy morning enhanced Thompson’s surreal impressions of the day, he wrote.
That mood intensified when he woke up in the operating room, mid-surgery, as the surgeons inserted the electrode. Lying with his head bolted to the stereotactic frame felt like “wearing a car grill,” he wrote. Overhearing his neurosurgeon chatting away on the other side of the plastic sheet draped around his head, Thompson was pleased to learn that he possesses a “robust thalamus.”
After placing the electrode, the doctors handed Thompson—still in the car grill—a pen and clipboard, as he had requested before the procedure. Without stimulation from the electrode now lodged in his brain, Thompson scratched out some squiggles meant to represent a brain and a spiral. The doctors turned on the juice and he tried again. Thompson could not see well without his glasses, but heard the medical team giggle at his drawings, so he figured the treatment had worked.
Cartoonist Richard Thompson penned this drawing—a brain saying, “Whee!”—during his DBS surgery, before (left) and after (right) the doctors turned on the juice. Image courtesy of Richard Thompson
“It was the strangest thing I had ever been through,” Thompson wrote. “Not altogether unpleasant, as I felt a bit spoiled by all the attention.”
A few months since the surgery, Thompson’s hair has grown back, and he was getting ready for his third programming session. At the first session, the effects of the stimulation were subtle, he told Alzforum. As the programmer adjusted the impulses, he might mumble or notice his smile go lopsided. The whole process took a few hours. But a week later, some of the shakiness returned. The second session was also a bit disappointing. “I’m hoping the third time is the charm,” he wrote.
“Wow! I Can Walk”
As for Duran, a few weeks after the electrode surgery, he returned to the hospital where De Salles implanted the impulse generator, which sends the stimulatory signals, into Duran’s chest. But Duran noticed benefits even before then. He and his wife thought he was imagining things when he saw, the morning after the first surgery, that his toes were not curled up tightly like on most mornings. In fact, De Salles expected something like this might happen. The GPi, having been prodded by the surgeons, was swollen and functioning poorly. Those relaxed toes were a good sign, indicating that disabling the GPi should reduce Duran’s symptoms.
This second surgery was shorter, but more difficult than the first, the Durans said. Duran acquired a series of staples marching down each side of his shaved scalp, and suffered more pain and a slower recovery. He underwent full anesthesia and did not know where he was when he woke up.
It was not until Duran had the stimulator turned on and programmed, at yet another appointment, that the benefits of DBS began to take full effect. His programmer held a remote control to his chest and amped the signal until Duran started tapping his feet and was able to traverse the hall with his walker. The effects felt the same as when his medication kicks in, Duran said.
The next morning, Duran hopped out of bed and walked into the bathroom unaided—he could barely make it there before the surgery. “Wow! I can walk … it’s real,” Duran told his wife. Brenda recalled, “We both just cried.”
Duran has dropped his daily pill count from 16 to 10, and hopes to go lower. His "on" periods last three to four hours, and he does not freeze up completely or fall over as the medications' effects wane. His only disappointment is that, just as he started to move better, the weather turned chilly, keeping him inside.
Memory Circuits
Could DBS one day become an equally accepted treatment for Alzheimer’s disease? Neurosurgeons have begun to test it. Unlike in Parkinson’s, where surgeons want to dampen undesirable signaling, doctors pursuing Alzheimer’s therapy want to increase activity. “It is like turning up the volume on a radio station,” said Andres Lozano of Toronto Western Hospital in Canada. Lozano is running a multicenter Phase 1 study, aiming to enroll 40 to 50 participants with mild AD to receive DBS to the fornix. “The fornix is the main pathway in and out of the memory circuit, so we thought that would be the best target,” he said.
In Lozano’s previous study of six people with AD, DBS was safe (see ARF related news story on Laxton et al., 2010). Some of the participants reported better memory and scored better on memory tests, but efficacy signals from such a small, open-label study mean little. One important finding from the pilot was that on positron emission tomography (PET) scans, the participants showed good glucose usage in the brain, said Constantine Lyketsos of Johns Hopkins University in Baltimore, Maryland. Normally, brain glucose metabolism decreases with progressing Alzheimer’s.
The Phase 2 study that Lozano and Lyketsos are now running is double-blinded; all subjects will receive electrodes, but only half will have them turned on. After a year, all participants will be able to receive stimulation. Lozano expects results from this study by 2015. He hopes that over 12 months, cognitive function will deteriorate more slowly in people with DBS than in those without. The team will also be investigating the rate of brain atrophy and blood flow.
Recruitment is going well, Lozano told Alzforum in an e-mail. His team in Toronto has operated on five patients since May 2012; Lyketsos’ Johns Hopkins group has performed two surgeries; and the Banner Alzheimer’s Institute in Phoenix, Arizona, has done one. The University of Florida in Gainesville will perform its first surgery this week. The Florida site has received some 40 inquiries from potential enrollees, according to Stacy Merritt at the university. “It is not easy to find the ideal candidate,” she wrote in an e-mail to Alzforum, because participants must fit into a specific range of cognitive and behavioral test scores. However, she is confident her site will enroll several subjects.
David Wolk and colleagues at the University of Pennsylvania in Philadelphia, another site, recently received institutional review board approval and are just getting started with recruitment. Brown University in Providence, Rhode Island, will also start enrolling subjects soon, according to Stephen Salloway at the University. Potential participants and their families find the concept of a “pacemaker for the brain” an appealing idea, he told Alzforum in an e-mail.
Lyketsos believes that the fornix is a good DBS target because it is affected early in the course of AD (Mielke et al., 2012); however, it is not the only possible bullseye for AD. Researchers at the University Hospital of Cologne, Germany, are testing DBS of the nucleus basalis of Meynert, one of the brain’s primary sources of acetylcholine. All current medications for Alzheimer’s work on the cholinergic system, noted Jens Kuhn, who is leading that study. He hopes to stimulate acetylcholine production with electricity, and thus improve memory and cognition. Kuhn is currently analyzing data from a pilot study of six people with mild AD, and expects to be able to conclude that the therapy is worthy of further investigation.
In their pilots, both Kuhn and Lozano noticed that younger people, with the mildest AD, saw the greatest benefit. Presumably, they had the most tissue left to save. In fact, DBS just might treat more than the symptoms of AD. Some studies have hinted that stimulation could cause growth factor production, Kuhn noted, which could help keep neurons alive. In a study of mice, Lozano and colleagues noticed that DBS enhanced neurogenesis in the hippocampus (see ARF related news story on Stone et al., 2011). “If this were to occur in humans, then there is a chance that the stimulation could alter the natural course of the illness,” Lozano said.—Amber Dance.
Comments
No Available Comments
Make a Comment
References
News Citations
- Deep-Brain Stimulation: Decade of Surgical Relief, Not Just for PD
- DBS Double Update: Call for Trial Registry, Two Targets Work for PD
- Zapping the Brain Can Help, But Where, and With What?
- DBS Update: Attempting to Stimulate Memory in Alzheimer’s
- Does Deep-Brain Stimulation Spark Neurogenesis, Enhance Learning?
Paper Citations
- Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, Starr PA, Simpson R, Baltuch G, De Salles A, Huang GD, Reda DJ, . Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med. 2010 Jun 3;362(22):2077-91. PubMed.
- Weaver FM, Follett KA, Stern M, Luo P, Harris C, Hur K, Marks WJ, Rothlind J, Sagher O, Moy C. Randomized trial of deep brain stimulation for Parkinson disease: Thirty-six-month outcomes. Neurology. 2012 Jun 20;
- Obeso JA, Rodríguez-Oroz MC, Rodríguez M, Arbizu J, Giménez-Amaya JM. The basal ganglia and disorders of movement: pathophysiological mechanisms. News Physiol Sci. 2002 Apr;17:51-5. PubMed.
- Gorgulho AA, Shields DC, Malkasian D, Behnke E, Desalles AA. Stereotactic coordinates associated with facial musculature contraction during high-frequency stimulation of the subthalamic nucleus. J Neurosurg. 2009 Jun;110(6):1317-21. PubMed.
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, Lozano AM. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol. 2010 Oct;68(4):521-34. PubMed.
- Mielke MM, Okonkwo OC, Oishi K, Mori S, Tighe S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer's disease. Alzheimers Dement. 2012 Mar;8(2):105-13. PubMed.
- Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, Frankland PW. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011 Sep 21;31(38):13469-84. PubMed.
Other Citations
External Citations
Further Reading
Papers
- Laxton AW, Lozano AM. Deep Brain Stimulation for the Treatment of Alzheimer Disease and Dementias. World Neurosurg. 2012 Jun 19; PubMed.
- Okun MS. Deep-brain stimulation for Parkinson's disease. N Engl J Med. 2012 Oct 18;367(16):1529-38. PubMed.
- Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol. 2011 Dec;68(12):1550-6. PubMed.
- Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, Wennberg RA, Lozano AM. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008 Jan;63(1):119-23. PubMed.
News
- Virtual Reality Reveals Deep-Brain Memory Stimulus, Early AD Signs
- Meynert, Oh, My! Deep Brain Stimulation to Treat Dementia?
- Deep-Brain Stimulation: Decade of Surgical Relief, Not Just for PD
- DBS Double Update: Call for Trial Registry, Two Targets Work for PD
- Zapping the Brain Can Help, But Where, and With What?
- DBS Update: Attempting to Stimulate Memory in Alzheimer’s
- Deep-Brain Stimulation Improves Connectivity in AD
- DBS Draws Consensus Nod for Parkinson’s, But Not Other Conditions
- PD Studies Highlight Deep Brain Stimulation, New Role for α-Synuclein
- Does Deep-Brain Stimulation Spark Neurogenesis, Enhance Learning?

 Paul Aisen
Paul Aisen  John (Wes) Ashford
John (Wes) Ashford 
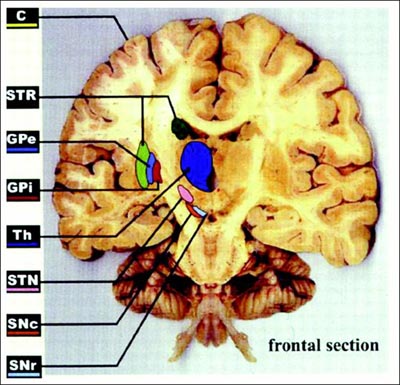


Comments
No Available Comments
Make a Comment
To make a comment you must login or register.