Researchers Join to Draw Posterior Cortical Atrophy Out of Shadows
Quick Links
You are a neurologist, and in comes a man in his fifties. He bumps into the doorframe before sitting down on the armrest of his chair, awkwardly trying to position his body in the seat. He can’t find the glass of water you place in front of him. Then he engages you in a perfectly coherent conversation about current events. Does this person have Alzheimer’s disease?
He may—sort of. But that’s not nearly the whole answer. Indeed, according to research clinicians who particularly care about patients like him, it is time to raise the profile of his peculiar and poorly understood condition. Two days before the official start of the Alzheimer’s Association International Conference held 14-19 July 2012 in Vancouver, Canada, Sebastian Crutch, Jonathan Schott, and Nick Fox of University College London, UK, together with Gil Rabinovici of the University of California, San Francisco, called the first in-person gathering of a newly formed working group on posterior cortical atrophy (PCA). The U.S. Alzheimer’s Association and Alzheimer's Research UK supported the meeting. The gathering drew a quorum of a larger group that had been forming over e-mail in the months before. More than 30 research clinicians from 20 centers in Britain, Canada, Australia, France, Germany, Holland, Italy, Spain, and the U.S. decided to move past their own idiosyncratic ways of defining PCA and see if they can accomplish more by working together. Each participating center has one to five dozen cases in its care.
The group’s to-do list is long. Besides pinning down how rare this neurodegenerative syndrome truly is, they aim to state a consensus about its core symptoms and boundaries to other diseases, and to develop diagnostic aids to be shared with the neurology community at large. They also agreed to pool samples for a genetic study of PCA’s peculiar features, and to do the groundwork for therapeutic trials specifically for these patients. “PCA is under-recognized and hugely untapped for research. It is strikingly different from typical AD,” said Schott.
PCA is a degenerative syndrome that affects the back of the brain. Some neurologists view PCA merely as atypical AD, where the disease’s amyloid deposits are distributed similarly to typical AD but its neurofibrillary tangles form more focally in occipital regions. In practice, however, PCA is essentially a clinical syndrome based on a "gestalt," as neurologists like to call it, of a collection of problems centered around visuospatial processing rather than memory.
Many patients’ initial symptoms largely fit that pattern. They cannot see objects they know are in front of them. They cannot construct in their mind’s eye a map of where things are relative to each other. For example, blind people can use a mobile phone because they retain a mental image of the keypad, but people with PCA lose that internal spatial representation. Sometimes static objects to them seem to be moving. Some symptoms are counterintuitive. One patient in Fox’s clinic, for example, recounted being unable to read the title of his daily newspaper when holding it in front of him on the train ride to work, while reading the small print just fine. Paradoxically, when looking down the train carriage, he could read the title on the same newspaper in the hands of a fellow commuter.
The symptoms can make patients disabled in daily life. Reading, writing, and calculation become difficult. People have to give up driving because they bump into things and misjudge the speed of other cars. They need help with mundane things such as finding light switches, putting a key into a lock, or picking out the can opener amid other gadgets in the drawer. “With a little help they can do almost everything; without help they can’t do anything,” Crutch recalled a carer saying.
At the same time, their memories are relatively intact, and most speak with acute insight into their condition. The British author Terry Pratchett, who eloquently raised awareness of AD through his own case, has a diagnosis of PCA. Even The Man Who Mistook His Wife for a Hat, from Oliver Sacks’ famous book, has since been speculated to have suffered from PCA. If it is ever appropriate to compare devastating diseases, PCA may be the slightly lesser evil than typical AD, and Pratchett, for his part, has said as much. “In our support group, PCA carers feel like the patient is still the person they have always known,” Crutch said.
Yet it’s important for clinical centers to develop support tailored specifically to PCA. People with this disease feel poorly served by traditional AD support groups. That is partly because they are younger and face different challenges, and partly because the activities offered in AD day centers—puzzles, large-font books—are visual and thus “actively unhelpful for people with PCA,” Crutch said. The UCL group runs regional support groups for some 200 patients with PCA in the UK and has a detailed website with information and patient videos.
The disease progresses slowly in some people and rapidly in others, but progress it does, and after some years, most patients do become memory-impaired and decline more broadly as their pathology spreads across the cortex.
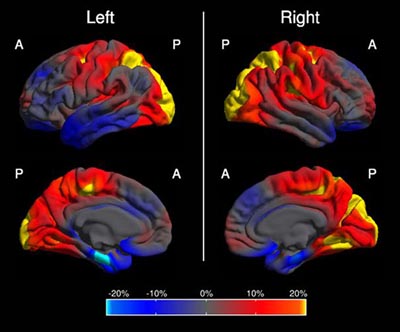
This image shows in which regions of the brain the cortex is abnormally thin in people with PCA compared to people with AD. The color scale represents the magnitude of the difference in cortical thickness; left and right refer to the brain's hemispheres. Yellow and red represent more cortical thinning in PCA (occipital and parietal cortex); blue represents more cortical thinning in AD (mediotemporal, entorhinal, frontal region). Summary image from 48 PCA and 30 AD cases, derived from Figure 1 in Lehmann et al., 2011.
How common is PCA? Are the researchers starting up a big effort for a smattering of cases? Probably not, Fox said. Determining the true prevalence and incidence of PCA requires that consistent diagnostic criteria be in place. Specialist centers have reported that 5 to 10 percent of their referrals may fit the picture, more among early onset cases. Anecdotally, when a research proposal Fox’s group had submitted to the UK Alzheimer’s Society was being discussed in a meeting with caregivers, the question arose whether this was too rare to warrant funding. “Suddenly, every layperson in the room said, ‘My husband has problems like that,’ or ‘The day center my wife goes to has someone like that,’” Fox said. From a pathology perspective, scientists know that some 25 percent of AD is atypical in terms of the distribution particularly of tangles. “We do not know what proportion of that is PCA. But even if it’s 5 percent, that is a lot of people,” Crutch said. In the U.S., that number would come to about 60,000.
That said, getting a PCA diagnosis can be a multiyear ordeal involving multiple visits to the ophthalmologist and prescription glasses until it’s clear the problem does not lie with the eyes, said Neil Graff-Radford of the Mayo Clinic, Jacksonville, Florida. Primary care providers may not see a PCA case in years, and general neurologists dealing mainly with older patients may not always recognize it. Because the symptoms are so unusual, some patients have been dismissed as malingering, Crutch said.
PCA is not new to the research community. Already in 1902, the Czech psychiatrist Arnold Pick mentioned a woman’s inability to see and grab a lit candle held in front of her face in his essay “Ueber eine eigenthuemliche sehstoerung senile dementer,” Jahrbuecher f. Psychiatrie u. Neurol., see excerpt. Several attempts at defining criteria exist. The neurologist Frank Benson at the University of California, Los Angeles, coined the term while describing the pattern of symptoms in five patients (Benson et al., 1988). Later, Mario Mendez, also of UCLA, proposed clinical diagnostic criteria arguing that PCA is its own syndrome, not just AD with visual symptoms (Mendez et al., 2002). Mendez was at the AAIC pre-meeting. Similar criteria came in 2004 from researchers at the Mayo Clinic in Rochester, including David Tang-Wai and Brad Boeve, who were both there as well (Tang-Wai et al., 2004). More recently, the International Working Group criteria (Dubois et al, 2007; Dubois et al., 2010), represented at the gathering by Bruno Dubois and Philip Scheltens, include PCA as an atypical variant of AD; they recommend diagnosis based on its core symptoms and biomarker evidence. These different sets of criteria are similar and complementary, but the group agreed that they lack detail and an agreed-upon grounding in biomarker findings.
There is a real need to collaborate, the group agreed. One problem is that, when reading another group’s papers about PCA or visuospatial variant AD, “one never knows for sure if these are the same types of patients as those at your own center,” said Fox. “We need to agree whom we are talking about.” Some research centers define PCA narrowly around its core visuospatial symptoms. Others also consider as potential PCA other cases that at first present with typically parietal symptoms such as difficulty with calculation or complex hand movements. These people may develop visual problems only years later. “They are in a diagnostic no man's land,” Crutch said. “If we want to understand AD as a whole, we need to grapple with the phenotypic continuum. PCA is not just a discrete subset. There is really no clear water between PCA and typical AD,” Crutch said.
Confused? Adding another twist to the diagnostic riddle is the fact that PCA is most often due to AD pathology, but not always. Its clinical symptoms occasionally show up as a consequence of other underlying diseases such as corticobasal degeneration, a tauopathy, or dementia with Lewy bodies. “The clinical syndromes are just constructs to describe the folks who come to see us, to help us categorize their problems,” Crutch said.
The limitations of the current clinical criteria make it difficult to replicate data that are beginning to come out on the neurobiology, biomarkers, and even immunological characteristics of PCA (e.g., Dorothée et al., 2012). They also hold back the design of new research.
Take genetics. Scientists want to know the genetic reasons for why some people get atrophy in the posterior cortex, why some get it early, and why some progress slowly and others quickly. At AAIC, Schott presented a poster on a small pilot genetic study of 58 people with PCA who are being seen at University College London, compared with 1,217 controls. Checking the loci of the AlzGene Top Results, the scientists found a different profile of effect size, even direction of effect in some cases, than these genes show in AD overall. In particular, this small study hints that in PCA, the gene ABCA7 may exert as high a risk as does ApoE. In AlzGene, ABCA7 occupies rank 4 to ApoE’s rank of 1.
The scientists need to replicate this finding with a larger group of patients, which would also allow for the possibility of identifying new genetic risks in GWAS. This requires pooling samples among the research groups working with PCA patients. Moreover, a tight definition of diagnostic criteria is important for genetic research. For example, the ABCA7 signal is stronger within a stringently defined subgroup of samples from people with core symptoms of PCA, and weaker in a larger group also including people whose symptoms place them toward the syndrome’s outer edges.
Or take clinical trials. Without clarity on exactly how to delineate PCA, how are trialists to define who should be allowed to participate in therapeutic studies? People with PCA are acutely aware of their disease and motivated to join trials, said Crutch, but there is no consistent approach at present. Some AD trials enroll people with PCA, as their criteria require no distinction among AD variants, but the protocol does not provide for their different phenotype. In those trials, PCA patients may create noise in the data. Some of the tests in those trials—visual memory, trail-making etc.—depend on skills PCA patients perform poorly to begin with because they cannot see the test properly. In contrast, they may ace auditory-based memory tests throughout the trial. Other trials exclude patients with PCA because they score too high on the MMSE, which is verbally driven.
“It seems unfair to exclude them because, based on their pathology, they are as likely to respond to the study medication as any AD patient. But where they are in trials, they fail tests for different reasons,” said Crutch. With a uniform definition of what PCA is, trialists could develop a subgroup analysis for PCA patients or develop treatment studies specifically for them, Crutch said.
Currently, there are no trials of disease-modifying compounds for PCA. A trial of donepezil in people with PCA suggested that these drugs are as modestly effective in them as in all people with AD, Crutch said. Even so, many clinicians do not prescribe these drugs to people with PCA in part because they often score well on the MMSE.
At their Vancouver meeting, the scientists decided to work toward crafting a consensus statement by the leading groups around the world on what they all agree constitutes the core and what the boundaries are of PCA. Importantly, they aim to integrate fluid biomarkers and brain imaging findings formally into the criteria for PCA. In particular, MRI and FDG-PET appear promising for the clinical syndrome of PCA. Amyloid and CSF biomarkers can be helpful in confirming that PCA is due to AD. They do not distinguish between PCA and typical AD since patients with both conditions have a similar, diffuse distribution of amyloid (Rosenbloom et al., 2011; Seguin et al., 2011). Amyloid plaques are known to start building up in the brain at least a decade before symptoms show, and scientists do not know whether the patterns of amyloid are different in the earliest, presymptomatic stages of PCA versus typical AD.
The scientists also brainstormed on collaborative projects. “We can make this group a platform to share our expertise and to study mechanistic questions of how PCA comes about, which is barely being done at all,” Rabinovici concluded. Emerging interest in PCA was already apparent throughout the main AAIC conference, with a string of presentations primarily on its brain imaging and clinical characteristics.—Gabrielle Strobel.
References
Paper Citations
- Lehmann M, Crutch SJ, Ridgway GR, Ridha BH, Barnes J, Warrington EK, Rossor MN, Fox NC. Cortical thickness and voxel-based morphometry in posterior cortical atrophy and typical Alzheimer's disease. Neurobiol Aging. 2011 Aug;32(8):1466-76. PubMed.
- Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988 Jul;45(7):789-93. PubMed.
- Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;14(1):33-40. PubMed.
- Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, Caselli RJ, Knopman DS, Petersen RC. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004 Oct 12;63(7):1168-74. PubMed.
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O'brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007 Aug;6(8):734-46. PubMed.
- Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O'brien J, Pasquier F, Robert P, Rossor M, Salloway S, Sarazin M, de Souza LC, Stern Y, Visser PJ, Scheltens P. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010 Nov;9(11):1118-27. PubMed.
- Dorothée G, Bottlaender M, Moukari E, de Souza LC, Maroy R, Corlier F, Colliot O, Chupin M, Lamari F, Lehéricy S, Dubois B, Sarazin M, Aucouturier P. Distinct Patterns of Antiamyloid-β Antibodies in Typical and Atypical Alzheimer Disease. Arch Neurol. 2012 Sep 1;69(9):1181-5. PubMed.
- Rosenbloom MH, Alkalay A, Agarwal N, Baker SL, O'Neil JP, Janabi M, Yen IV, Growdon M, Jang J, Madison C, Mormino EC, Rosen HJ, Gorno-Tempini ML, Weiner MW, Miller BL, Jagust WJ, Rabinovici GD. Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology. 2011 May 24;76(21):1789-96. PubMed.
- Seguin J, Formaglio M, Perret-Liaudet A, Quadrio I, Tholance Y, Rouaud O, Thomas-Anterion C, Croisile B, Mollion H, Moreaud O, Salzmann M, Dorey A, Bataillard M, Coste MH, Vighetto A, Krolak-Salmon P. CSF biomarkers in posterior cortical atrophy. Neurology. 2011 May 24;76(21):1782-8. PubMed.
External Citations
Further Reading
Papers
- Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet Neurol. 2012 Feb;11(2):170-8. PubMed.
- Lehmann M, Barnes J, Ridgway GR, Ryan NS, Warrington EK, Crutch SJ, Fox NC. Global gray matter changes in posterior cortical atrophy: A serial imaging study. Alzheimers Dement. 2012 Feb 23; PubMed.
- Wolk DA, Price JC, Madeira C, Saxton JA, Snitz BE, Lopez OL, Mathis CA, Klunk WE, Dekosky ST. Amyloid imaging in dementias with atypical presentation. Alzheimers Dement. 2012 Jan 26; PubMed.
- Sugimoto A, Midorikawa A, Koyama S, Futamura A, Hieda S, Kawamura M. Picture agnosia as a characteristic of posterior cortical atrophy. Eur Neurol. 2012;68(1):34-41. PubMed.
- Pelak VS, Smyth SF, Boyer PJ, Filley CM. Computerized visual field defects in posterior cortical atrophy. Neurology. 2011 Dec 13;77(24):2119-22. PubMed.
- Warren JD, Fletcher PD, Golden HL. The paradox of syndromic diversity in Alzheimer disease. Nat Rev Neurol. 2012 Aug;8(8):451-64. PubMed.
News
- Natural Antibodies in AD Patients: Are Some Protective?
- Experimental α7 Agonist Meets Cognitive and Clinical Endpoints
- New Assays for Aβ Oligomers in CSF Claim Femtogram Sensitivity
- Q&A With Roche’s CNS Leader Luca Santarelli
- Wave of New BACE Inhibitors Heading to Phase 2
- When Is a C9ORF72 Repeat Expansion Not a C9ORF72 Repeat Expansion?
Annotate
To make an annotation you must Login or Register.

Comments
�
I would like to know if there are any facilities currently taking PCA-diagnosed patients for observation and study of this disease. My wife is now 61 and has been diagnosed with PCA. We started seeing symptoms about two years ago but the disease has progressed rapidly. She currently struggles to pick up any object, and can't handle any type of stairs without holding on to a person or fear of falling. She paces almost constantly. I have tried to document her rapid changes for doctors in Chapel Hill. She was formerly a registered nurse with great talent for painting, sewing, decorating, etc. She has never been a smoker or alcohol user and has no known hereditary issues. Her request in the past had always been to use her body for science and research should there be an opportunity. She is now being recommended for placement in a memory care facility and I see this as opportunity should there be any place looking to research this disease. As her legal guardian and power of attorney, I would welcome the opportunity to discuss any possibilities that would help fulfill her requests.
My mother is 10 years into this disease. I knew she was sick when she couldn't plug the telephone cord into the wall. She also had difficulty aiming the metal part into the base when putting her seat belt on. Neurologists said they couldn't find anything for three years but I knew. As we went to Los Angeles, the neurologists there had had three cases already and they knew right away.
My mom was a doctor. She could still discuss complex topics. Her depth perception and visual fields were definitely affected. I had to take away her license. The doctors never suggested this and other family members were opposed to it initially. I knew she could have run over someone. She was scared to use stairs and had difficulty walking in general. She couldn't walk in a given direction and needed to be guided.
Eventually she began forgetting words and describing objects so that we could name them for her. Now she greatly struggles to swallow and is wheelchair bound and uncomfortable. Insurance for getting wheelchair and other goods has been a incredibly difficult with a rare diagnosis. She doesn't speak anymore. The occasional word or laugh comes out so that you know she understands the conversation. At other times, she seems completely gone. Sometimes she recognizes me sometimes she doesn't. When she was in the ER, I didn't think she recognized me but the nurse said that she was much more calm with me and my sister in the room.
Nothing can prepare you for how difficult this disease is. Some friends don't seem to believe my assessment of her and minimize it. My mom is still able to smell and taste....the only joys left. If I put a gardenia, lavender, scratch a lemon peel under her nose, she is in heaven. A hug and touch in general...gently stroking her forehead....gentle voice. Touch is so important in ill people....they get less of it because others are less willing to touch ill people and because they are unable to reach out and touch others....but it is so important....look at the studies with babies and touch.
I hope they continue with research on this disease. It is such and incredibly sad illness.
Make a Comment
To make a comment you must login or register.