Self-Derived Stem Cells Fly Under Monkey’s Immune Radar
Quick Links
Induced pluripotent stem cells (iPSCs) offer the tantalizing prospect that with some manipulation, skin cells could one day help replace dying dopamine neurons in the brains of people with Parkinson's disease. However, scientists are still working out how to avoid an immune reaction. Do cells from another donor carry a greater risk of attack by the immune system than the patient's own? The question is controversial, and studies have never compared iPSC cell immunogenicity side-by-side. Now, in a paper published online today in Stem Cell Reports, scientists led by Jun Takahashi of Kyoto University, Japan, make this direct comparison in macaque monkeys. They report that implanted dopamine neurons derived from an animal's own cells provoke a much smaller immune response than did cells from other monkeys, allowing more of the transplanted neurons to survive.
"This work gives us a roadmap for the best paths to pursue," said Ole Isacson of Harvard Medical School’s McLean Hospital, Belmont, Massachusetts, who was not involved in the project. "Immune regulation is important, and may guide us to better cell therapy."
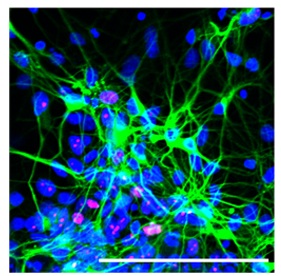
Neurons (green) and some neural progenitor cells (pink) derived from macaque iPSCs Image credit: Morizane et al., 2013/Stem Cell Reports
In clinical studies that started back in 1987, researchers implanted dopaminergic neurons from developing fetuses into the brains of Parkinson's patients with some success (for a review, see Lindvall and Bjorklund, 2004). However, the use of embryonic stem cells is mired in controversy and they generate only tiny quantities of dopaminergic neurons, hence scientists have turned to iPSCs as a source. With those cells, one pressing question is how individualized they need to be. Can they come from another donor, or do they have to come from a patient's own cells to avoid an immune attack? Previous studies suggested that both types of graft could survive in non-human primates (see Emborg et al., 2013 and Redmond et al., 2008), but no studies directly compared the two to see if one is easier on the immune system.
To find out, lead author Asuka Morizane and colleagues derived iPSCs from peripheral blood or skin cells of four cynomolgus monkeys using either retroviral vectors that integrate into the recipient cells' genome, or episomal vectors that stay separate. In 2012, co-author Shinya Yamanaka, also at Kyoto University, received the Nobel prize for his 2006 discovery that just four transcription factors could reprogram mouse fibroblasts to a pluripotent state (see Takahashi and Yamanaka, 2006). He and colleagues later modified the method to avoid using integrating retroviral vectors (see ARF related news story). In this study, Yamanaka helped establish the monkey iPSCs.
To coax these cells into becoming neurons, the researchers incubated them with inhibitors of bone morphogenetic protein and Activin/NODAL signaling. Adding purmorphamine/FGF8 and FGF2/FGF20 led specifically to dopamine neurons. Morizane and colleagues then injected those neurons into the left striatum of either the original donor monkey in what is called an "autograft," or into one of four different monkeys in an "allograft." For up to four months, the researchers observed the animals' immune responses to the new cells without giving them immunosuppressing drugs.
Allografts elicited the stronger immune reaction. Positron emission tomography with PK11195 revealed microglial activation in the brain in one case. In addition, major histocompatibility complex (MHC)-II and IBA1 positivity signaled stronger activation of microglia in the allograft recipients. More T cells, indicated by CD45 and CD3 staining, migrated to these grafts. Still, transplants were not rejected outright in these animals; about half the allografted neurons survived compared to monkeys that received autografts. In the autografted animals, minimal inflammation showed up mainly along the path where the injection needle had penetrated. Taken together, the results suggest that while allografts would require some immunosuppression, autografts would not, wrote the authors.
Despite the added cost, it seems that self-derived dopamine cells would serve PD patients best, said Jeffrey Kordower, Rush University, Chicago, Illinois, adding that a milder immune reaction probably means more functional grafts. However, he pointed out that scientists have yet to determine whether dopamine cells derived from PD patients would survive and function as well as those from healthy people. Researchers are working to answer that question in cell cultures, he said. Kordower also noted that the primates used in the current study did not model PD, so it is unclear whether these grafts would alleviate problems associated with the disease. What's more, he remarked on the lack of outgrowth and innervation of the monkeys' transplanted neurons. This could either mean that more time is needed for the outgrowth to occur, or that added factors will have to help these neurons functionally integrate into host tissue.
Takahashi said his group plans to examine the function of the patient-derived dopaminergic neurons in monkeys for longer periods of time. He and colleagues previously reported that allogenic transplants from monkey embryonic stem cells alleviated symptoms in primate models of PD (see Takagi et al., 2005).
Isacson's own work, too, suggests that autografts will be better in the long run. He noted that the technology will likely become cheaper and easier. At the same time, Isacson did not discount allografts. After all, the early fetal cell transplants in humans came from separate donors and had some benefit, he said. This study suggests that well-controlled immune suppression could help allografts survive, Takahashi wrote to Alzforum in an email. One way to lessen their immunogenicity may be to match human leukocyte antigen (HLA) haplotypes—patterns of protein markers that form a ‘fingerprint’ for each person's cells to identify them as self—between donor and host, he added. Some groups propose creating iPSC banks that will stock cell lines of varying haplotypes to supply a large population (see Taylor et al., 2011).—Gwyneth Dickey Zakaib
References
News Citations
Paper Citations
- Lindvall O, Björklund A. Cell therapy in Parkinson's disease. NeuroRx. 2004 Oct;1(4):382-93. PubMed.
- Emborg ME, Liu Y, Xi J, Zhang X, Yin Y, Lu J, Joers V, Swanson C, Holden JE, Zhang SC. Induced pluripotent stem cell-derived neural cells survive and mature in the nonhuman primate brain. Cell Rep. 2013 Mar 28;3(3):646-50. PubMed.
- Redmond DE, Vinuela A, Kordower JH, Isacson O. Influence of cell preparation and target location on the behavioral recovery after striatal transplantation of fetal dopaminergic neurons in a primate model of Parkinson's disease. Neurobiol Dis. 2008 Jan;29(1):103-16. PubMed.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663-76. PubMed.
- Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005 Jan;115(1):102-9. PubMed.
- Taylor CJ, Bolton EM, Bradley JA. Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc Lond B Biol Sci. 2011 Aug 12;366(1575):2312-22. PubMed.
Further Reading
Papers
- Bjugstad KB, Teng YD, Redmond DE, Elsworth JD, Roth RH, Cornelius SK, Snyder EY, Sladek JR. Human neural stem cells migrate along the nigrostriatal pathway in a primate model of Parkinson's disease. Exp Neurol. 2008 Jun;211(2):362-9. PubMed.
- Lindvall O, Björklund A. Cell therapy in Parkinson's disease. NeuroRx. 2004 Oct;1(4):382-93. PubMed.
- Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005 Jan;115(1):102-9. PubMed.
- Cao J, Li X, Lu X, Zhang C, Yu H, Zhao T. Cells derived from iPSC can be immunogenic - Yes or No?. Protein Cell. 2013 Sep 3; PubMed.
- Kaneko S, Yamanaka S. To be immunogenic, or not to be: that's the iPSC question. Cell Stem Cell. 2013 Apr 4;12(4):385-6. PubMed.
- Faiz M, Nagy A. Induced Pluripotent Stem Cells and Disorders of the Nervous System: Progress, Problems, and Prospects. Neuroscientist. 2013 Jun 24; PubMed.
- Emborg ME, Liu Y, Xi J, Zhang X, Yin Y, Lu J, Joers V, Swanson C, Holden JE, Zhang SC. Induced pluripotent stem cell-derived neural cells survive and mature in the nonhuman primate brain. Cell Rep. 2013 Mar 28;3(3):646-50. PubMed.
- Redmond DE, Vinuela A, Kordower JH, Isacson O. Influence of cell preparation and target location on the behavioral recovery after striatal transplantation of fetal dopaminergic neurons in a primate model of Parkinson's disease. Neurobiol Dis. 2008 Jan;29(1):103-16. PubMed.
- Taylor CJ, Bolton EM, Bradley JA. Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc Lond B Biol Sci. 2011 Aug 12;366(1575):2312-22. PubMed.
News
- Stem Cells Treat Epileptic Symptoms in Mice
- Cholinergic Neurons From Stem Cells Rescue Mouse Memories
- Stem Cells: Simpler to Make, Easier on the Immune System
- Therapeutic-Grade Dopaminergic Neurons From Stem Cells?
- Faster, Safer Ways to Cook Up Dopaminergic Neurons
- Not So Fast: iPS Cells Have Potential Pitfalls
- In Alzheimer Disease Research, iPS Cells Catch On Slowly
Primary Papers
- Morizane A, Doi D, Kikuchi T, Okita K, Hotta A, Kawasaki T, Hayashi T, Onoe H, Shiina T, Yamanaka S, Takahashi J. Direct Comparison of Autologous and Allogeneic Transplantation of iPSC-Derived Neural Cells in the Brain of a Nonhuman Primate. Stem Cell Reports. 2013;1(4):283-92. Epub 2013 Sep 26 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.