Prion Stabilizers Boost Survival in Infected Mice—What About Other Proteinopathies?
Quick Links
If you can’t beat 'em, keep them joined? That is the basis of a new therapy that stops the spread of prions in its tracks. As described August 5 in Science Translational Medicine, researchers have developed polythiophene compounds that stabilize prion oligomers, preventing them from splitting into new seeds of destruction. The lead compound, LIN5044, prolonged survival in prion-infected mice by up to two months when given prior to infection, and added a couple of weeks to the animals’ lives when administered after symptoms kicked in. The researchers proposed that polythiophenes could have a similar effect on other amyloidogenic proteins, such as Aβ and tau.
“This study is really a poster child of how to go about rational drug design,” said David Teplow of the University of California, Los Angeles. “Everyone talks about doing it, but few actually do.”
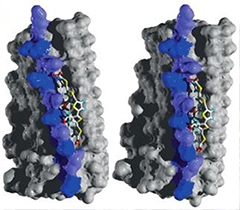
Sticky Stabilizers.
Carboxyl groups on LIN5001 bind lysine residues (blue and purple) on a stack of HET-s prion proteins, stabilizing the prion. [Courtesy of Herrmann et al., Science Translational Medicine, 2015.]
Prions are misfolded proteins that propagate by corrupting properly folded versions of themselves. The prion protein PrP (known as PrPSC in its misfolded form) causes deadly transmissible spongiform encephalopathies, including bovine spongiform encephalopathy (BSE) in cows, scrapie in sheep, and Creutzfeldt-Jakob disease (CJD) in humans. PrPSC molecules attach to and convert normal PrP proteins into the toxic form, accelerating the formation of PrPSC aggregates and fibrils. When these aggregates break apart, each fragment becomes a seed that can corrupt new PrP molecules. This break-up process is what senior author Adriano Aguzzi of the University of Zurich aimed to target. “That is the most important moment in the life of a prion fibril, just like mitosis is for a cancer cell,” Aguzzi told Alzforum.
As his weapon of choice, Aguzzi chose a group of compounds already known for their amyloid-smothering properties. Luminescent conjugated polythiophenes (LCPs) are fluorescent molecules that preferentially latch onto the cross β-sheet structures common to all amyloids. LCPs label PrPSC molecules with higher specificity and sensitivity than Congo Red, a popular amyloid dye. In 2012, Aguzzi and colleagues reported that LCPs stabilized prions in brain slices from prion-infected mice, and reduced their infectivity in vitro (see Margalith et al., 2012).
Now, first author Uli Herrmann and colleagues have improved LCPs and shown they can work in animals. The researchers started by continuously infusing a mixture of four candidate LCPs through a minipump implanted in the brains of tga20 mice, which overexpress normal PrP. One week after starting the infusion, the researchers infected the mice with prions of the Rocky Mountain Lab (RML) strain. The LCP mix prolonged survival by 25 percent. Why? It turned out the treated mice harbored more prion particles that resisted boiling in detergent than untreated mice did. However, the LCP-treated mice had fewer infectious prion units. The researchers hypothesized that LCP treatment stabilized the prions, which prevented them from breaking apart and forming new infectious seeds.
Given the anti-prion activity of the LCP mix, the researchers next tried the compounds one by one. They found that when given to mice before they were inoculated, LIN5001, an LCP with five thiophene rings, worked better than any other. It prolonged survival by 36.5 percent: Mice pretreated with the compound survived for an average of 90 days after infection, while their untreated counterparts died after 66 days. This compound also extended the lives of mice by more than 15 days when administered three weeks after prion infection, when mice are still asymptomatic. When given to fully symptomatic mice 50 days after prion infection, LIN5001 gave mice a hint of a benefit.
The researchers then used density gradient centrifugation to separate out the prions from infected mouse brains by size, and found that LIN5001-treated mice had only small prion aggregates, whereas mice treated with LIN5002 (an ineffective compound) had both large and small. They hypothesized that LIN5001 slowed prion spread by binding to PrPSC early in the aggregation process, when prion clusters were still fledglings.
To get a better idea of how LIN5001 bound to prions, the researchers mixed the compound with the fungal prion HET-s, which is the only self-propagating amyloid whose atomic structure has been solved. Using solid-state NMR spectroscopy, the researchers found that carboxyl groups on LIN5001 linked up with the positively charged lysine residues on HET-s aggregates. These interactions were spaced at regular 5- and 10-Angstrom intervals. The researchers then fed in this complementarity information into a molecular dynamics simulation, which generated a plausible structure of LIN5001 interacting with HET-s (see image above).
The researchers used information from the simulations to pick out different LCPs from their library that would stabilize prions even better than LIN5001 did. They tested six of these compounds in mice. The winner—LIN5044—stabilized prions most efficiently and had the largest anti-prion effect in mice. When administered prior to infection, it prolonged survival of prion-infected mice by 87.5 percent (56 days). When given 50 days after infection, when the mice already had severe motor symptoms, the compound prolonged survival by 22 percent (14 days) when administered intracerebrally and by 14.5 percent (nine days) when injected into the peritoneal cavity, indicating that the compound crosses the blood-brain barrier to some extent. LIN5044 also worked against a different strain of prion—263K—extending survival by 13 percent when administered to mice expressing the normal version of that protein.
Histological analysis of brain sections taken 63 days after infection revealed profound neuronal loss and an accumulation of activated astrocytes in hippocampal slices from untreated mice, whereas this damage was greatly reduced or absent in mice pretreated with LIN5044. This indicated that the compound slowed the process of neurodegeneration and inflammation. Homogenates from the pretreated mice also failed to fibrillarize PrP proteins in vivo, whereas those from untreated mice converted the proteins to fibrils.
While it has not been formally tested, Aguzzi hypothesized that LIN5044 would also stabilize other amyloidogenic proteins, including Aβ, tau, and α-synuclein. Teplow commented that other amyloidogenic proteins have a similar cross β-structure as prions, and suggested researchers give the LCPs a try in APP mouse models. Mathias Jucker of the University of Tübingen in Germany commented that his lab had previously tried this with peripheral injections of LCPs and did not see a therapeutic effect. However, now that Aguzzi and colleagues have greatly improved the compounds, Jucker hoped it could work against an array of proteinopathies.
Lary Walker of Emory University in Atlanta expressed optimism that LCPs could work as a treatment for AD, if indeed propagation of Aβ is the pathogenic mechanism, as many researchers propose. He and Teplow were impressed that the researchers could prolong survival in sick prion-infected mice, as treatments would likely be given to AD patients long after the putative propagation process had begun. However, he cautioned that the path to drug development is a long one.
Aguzzi said his lab’s role in the development of LCPs as drugs is finished now, and added that his lab plans to use the compounds as tools to label prions and study their spread throughout the brain. It will be up to pharmaceutical companies to move LCPs forward from here, he said.—Jessica Shugart
References
Paper Citations
- Margalith I, Suter C, Ballmer B, Schwarz P, Tiberi C, Sonati T, Falsig J, Nyström S, Hammarström P, Aslund A, Nilsson KP, Yam A, Whitters E, Hornemann S, Aguzzi A. Polythiophenes inhibit prion propagation by stabilizing prion protein (PrP) aggregates. J Biol Chem. 2012 Jun 1;287(23):18872-87. Epub 2012 Apr 6 PubMed.
Further Reading
Papers
- Goedert M. NEURODEGENERATION. Alzheimer's and Parkinson's diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015 Aug 7;349(6248):1255555. PubMed.
Primary Papers
- Herrmann US, Schütz AK, Shirani H, Huang D, Saban D, Nuvolone M, Li B, Ballmer B, Åslund AK, Mason JJ, Rushing E, Budka H, Nyström S, Hammarström P, Böckmann A, Caflisch A, Meier BH, Nilsson KP, Hornemann S, Aguzzi A. Structure-based drug design identifies polythiophenes as antiprion compounds. Sci Transl Med. 2015 Aug 5;7(299):299ra123. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.