Not Seeing Eye to Eye: Do Lenses Accumulate Aβ?
Quick Links
What if you could diagnose Alzheimer’s disease at an early stage by examining a person’s eyes? In 2003, researchers led by Lee Goldstein at Boston University, Massachusetts, proposed just such a possibility when they reported that Aβ deposits form cataracts in the lenses of people with AD (see Goldstein et al., 2003). The finding made the national news and raised hopes of a simple test. It also excited many scientists in the Alzheimer’s field. Since the eye is easily accessible by non-invasive means, the method seemed a low-hanging fruit for a diagnostic. In the decade since, however, other labs have struggled to corroborate the finding, and no independent replication was published. Recently, in the January issue of Experimental Eye Research, scientists led by Ralph Michael at the University Autònoma of Barcelona, Spain, reported that they were unable to find any sign of Aβ in the lenses of AD patients using immunohistochemical and amyloid stains. The new data do not rule out the possibility that Aβ is there, but, together with a lack of independent replication, cast doubt on the usefulness of the lens as a diagnostic tissue, some researchers suggest. Others believe that Goldstein’s methods were rigorous and that Aβ does exist in the lens. The field may soon see clearly on this, as several human trials gearing up this year will test lens Aβ as a diagnostic for AD. Meanwhile, a startup company based in Cambridge, Massachusetts, is evaluating a different eye-based diagnostic, which purports to detect AD through a pupillary response.
“There are many reasons why [data] might not be reproducible outside the originating lab,” Bill Klunk at the University of Pittsburgh, Pennsylvania, wrote to Alzforum. “Failure to reproduce doesn't necessarily make the original findings wrong, but it does raise issues about the robustness and reliability of the findings for widespread use.” Likewise, Maria-José Tassignon, who heads the ophthalmology department at Antwerp University Hospital, Belgium, noted, “In medicine, a test is only attractive if it’s quickly done and reliable.” Other scientists insisted on speaking off the record but suggested that if the method worked, there could by now be sessions on lens Aβ at AD conferences.
Goldstein asserts he now has a quick and reliable method to spot deposits. It comes in the form of an optical device that he says detects small Aβ aggregates in clear lenses via their light-scattering properties. Goldstein contends that analytical methods such as mass spectrometry have proven the existence of Aβ deposits in AD lenses, and that the only remaining question is whether these deposits can be easily detected in living people. He hopes to settle this question in upcoming trials of his quasi-elastic light-scattering (QLS) device. Developed at BU, the instrument is a “custom confocal QLS-enabled scanning laser ophthalmoscope,” Goldstein said.
The medical technology company Cognoptix (formerly called Neuroptix), in Acton, Massachusetts, is taking a different Aβ-based eye scanning method into clinical trials. Goldstein co-founded Neuroptix in 2001 but is no longer affiliated with it, according to both Goldstein and Cognoptix. Using technology acquired from the University of California, San Diego, Cognoptix researchers place an ointment in the eye that they say binds to Aβ deposits to produce a fluorescent signal visible by laser scans (see Cao et al., 2012). Called “SAPPHIRE II,” this test distinguished five AD patients from five healthy controls in a small proof-of-concept trial, the company claims. The work is unpublished, but CEO Paul Hartung told Alzforum he will present the data at the Alzheimer’s Association International Conference to be held this July in Boston, Massachusetts. In February, Cognoptix began a trial of 20 patients and 20 controls that will compare their method to clinical diagnoses and PET amyloid imaging (see company press release). Hartung expects that trial to conclude in about three months. Hartung noted that the company chose the probe technology over QLS because the latter, while extremely sensitive, was not specific enough. The light-scattering method was unable to identify lens aggregates as being Aβ, and therefore did not distinguish AD from other medical conditions, Hartung said. In 2006, Cognoptix ran a Phase 1 trial of QLS technology using a QLS device developed by the company (see ScienceDaily story).
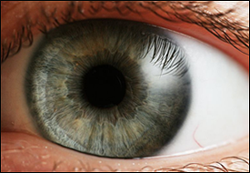
Eye-based diagnostic for Alzheimer's—is it in the offing? Image courtesy of Wikimedia Commons
While these groups are beginning human trials, other researchers have been unsuccessful at replicating the original publication. Michael is the only researcher to publish negative results. He told Alzforum he initially found the method tantalizing. He attempted to replicate Goldstein’s findings, which are based on lens tissue from nine AD patients and eight controls, by examining lenses donated to an eye bank by 17 clinically diagnosed AD patients, four neuropathologically confirmed AD patients, and 15 age-matched controls. Six of the AD donors and seven of the controls in Michael’s study had visible cataracts. (Different types of cataracts exist in aging eyes; Goldstein’s original work focused on a type that is not visible to the naked eye because it is behind the iris: see ARF related news story.) Michael and colleagues found no evidence of Aβ deposits in any of the 54 lenses from 36 people analyzed in this study. They tried Congo red and thioflavin stains, which detect amyloid as well as antibodies specific for Aβ. As a positive staining control, the researchers used brain tissue from AD patients and a cornea from a donor with lattice corneal dystrophy, which produces amyloid deposits. When initial results were negative, they repeated Congo red staining of AD lenses using a more sensitive staining protocol (Romhanyi protocol instead of Puchtler; see Bély and Makovitzky, 2006), but still saw nothing. They sent some lenses to an independent laboratory at Emory University, Atlanta, Georgia, which also found nothing. “It’s a bit disappointing that in our hands, it is not possible to use the lens as an early indicator for AD,” Michael told Alzforum.
The researchers speculated on possible reasons for the discrepancy between their results and Goldstein’s findings. They noted that some of Goldstein’s techniques, such as glutaraldehyde fixation and the use of Canada balsam and alcohol rinses during Congo red staining with Bennhold’s protocol, might have produced false-positive results. Lenses contain many proteins that form fibrillar β-pleated sheets and might produce a non-specific signal, Michael said. Michael continues to study the issue with more sophisticated techniques. In future work, he plans to use nuclear magnetic resonance (NMR) spectroscopy to look for conformational protein changes in AD lenses, he told Alzforum. He believes other researchers have also failed to find Aβ deposits in the lens. “A lot of people have looked, but because they found negative results, have not published. Some colleagues told me in conversation that they made a small test but did not continue,” Michael said.
Tassignon is one such researcher. She told Alzforum she performed initial studies with human lenses, but found nothing and stopped. She does not consider her tests definitive, and thus never published. Perhaps there is Aβ in the lens, but if it is difficult to find, it may not be a useful diagnostic, she suggested. On the other hand, Sabina Janciauskiene, a professor of experimental medicine at Hannover Medical School, Germany, believes Aβ in the eye does not reflect specific disease signatures. Janciauskiene performed a study of Aβ levels in the aqueous humor of the eye in people with cataracts and other eye diseases such as diabetic retinopathy and macular degeneration (see Janciauskiene et al., 2011). She found the peptide was present at low levels but did not associate with particular diseases. However, she studied no AD patients. Of Goldstein’s work, she said, “This story was very exciting. I think many people looked originally, but nothing much came after that.”
Other researchers find Goldstein’s data convincing. Barry Greenberg at the Toronto Dementia Research Alliance, Canada, has seen unpublished data and believes the work is solid. He pointed out that Goldstein identified lens Aβ using a variety of methods, including immunogold electron microscopy and two forms of mass spectrometry. The missing piece is confirmation in larger samples, Greenberg said. Greenberg himself is leading a clinical trial of Goldstein’s QLS technology, and expects to start enrolling patients this year. Participants will have various types of dementia, including AD, dementia with Lewy bodies, MCI, and vascular cognitive impairment; some will be healthy controls. The goal is to see how sensitive and specific the technique is at picking up Alzheimer’s. In a separate study, Greenberg will look at lenses from neuropathologically confirmed cases of AD.
John Clark at the University of Washington in Seattle also collaborates with Goldstein to test the technology. He is finalizing approvals for a trial to begin this year that will correlate lens scan results with clinical diagnosis and PET amyloid imaging in about 100 AD patients and controls. Clark told Alzforum that in preliminary data on his patient population, he sees results consistent with Goldstein’s hypothesis. The lenses of AD patients scatter more light than do control lenses in the region where Goldstein reports Aβ deposits. Clark sees high variability among patients, however. He hopes more data on a larger population will reveal whether this method can serve as a useful diagnostic. Clark considers the results in the paper by Michael et al. not definitive, because the authors did not use analytical techniques such as mass spectroscopy. “Congo red and thioflavin staining are variable under the best of conditions,” Clark said.
For his part, Goldstein notes that old lenses are one of the most difficult tissues in the body to stain. The tissue is hard and contains numerous packed fibrillar deposits. It took his group years to develop their lens staining techniques, Goldstein told Alzforum. He believes Michael and other researchers may be missing Aβ deposits due to these technical hurdles. Goldstein pointed out that Michael and colleagues lacked a positive control lens to be sure their techniques worked in this tissue. Michael disputes the idea that lenses require special staining techniques. He said he used similar techniques as Goldstein, but with some differences such as the method of fixation. He said that while having a positive control lens would be ideal, there is no way to get one if these tissues accumulate no amyloid.
Goldstein said his QLS instrument gets around troublesome staining issues. He claimed the method is reliable and “exceedingly sensitive.” It is described in a 2010 paper reporting the presence of Aβ in postmortem lenses from 16 people with Down’s syndrome, one as young as two years old (see ARF related news story on Moncaster et al., 2010). QLS picks up the presence of small, 3-30 nm Aβ aggregates in clear lenses, long before cataracts form, Goldstein said, adding that he is preparing to publish data showing his method works in Tg2576 AD mice.
David Hunter, an ophthalmologist at Boston Children’s Hospital and a coauthor on the paper by Moncaster et al., is following up on the Down’s syndrome study by scanning the eyes of children with the condition and comparing them to controls. He wants to see whether Aβ accumulates in the lens with age and could serve as a marker of AD-like changes in this population. The initial study will be cross-sectional, but he hopes to follow participants longitudinally as well, he said. Local eye research foundations fund Hunter’s work. None of the planned QLS trials have received corporate sponsorship, Goldstein noted.
Pierre Tariot at Banner Alzheimer’s Institute, Phoenix, Arizona, is a clinical advisor to Cognoptix and an investigator in their upcoming trial. “I was impressed that Goldstein’s approach confirmed the presence of Aβ by multiple techniques,” he told Alzforum. He believes technical differences may underlie the lack of replication in the recent study, adding, “I’m reasonably swayed by the animal model data and the minimal preliminary data Cognoptix has presented. It’s a plausible approach that deserves to be tested further, which is why we chose to participate.” Murali Doraiswamy at Duke University, Durham, North Carolina, and also an advisor to Cognoptix, agreed. “I still think [the technique] is promising, but ultimately the proof will have to come from prospective human validation trials,” Doraiswamy wrote to Alzforum.
Meanwhile, Leonard Scinto, who heads Cambridge Neurodiagnostics, based in Massachusetts and London, U.K., has developed a different putative diagnostic for AD that also relies on the eye. In 1994, Scinto, then working at Harvard Medical School with Huntington Potter, reported exaggerated dilation in the pupils of AD patients in response to the topical agent tropicamide, a cholinergic antagonist similar to the better known atropine (see Scinto et al., 1994). This response is due to “the ravages of tau pathology in the midbrain” and is greater in people who carry an ApoE4 allele, Scinto claimed (see Scinto et al., 1999; Scinto et al., 2001; Scinto, 2007). Later work suggested the test could predict cognitive decline (see Scinto, 2008). Other researchers have obtained mixed results, with some reporting that the method works and others that it does not (see, e.g., Litvan and FitzGibbon, 1996; FitzSimon et al., 1997; Iijima et al., 2003; and Mahmoudian et al., 2009). Scinto will evaluate it in Phase 2b and Phase 3 trials to begin later this year in the U.K. and Europe. The former will comprise 150 AD patients and age-matched controls, while the latter will be composed of almost 200 participants, he told Alzforum. Scinto declined to say who is funding the trials.—Madolyn Bowman Rogers
References
News Citations
- Orlando: Eyeballing the Eye in the Hunt for That Elusive Prize, an AD Biomarker
- New Findings Offer Hope for Down Syndrome
Paper Citations
- Goldstein LE, Muffat JA, Cherny RA, Moir RD, Ericsson MH, Huang X, Mavros C, Coccia JA, Faget KY, Fitch KA, Masters CL, Tanzi RE, Chylack LT, Bush AI. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer's disease. Lancet. 2003 Apr 12;361(9365):1258-65. PubMed.
- Cao K, Farahi M, Dakanali M, Chang WM, Sigurdson CJ, Theodorakis EA, Yang J. Aminonaphthalene 2-cyanoacrylate (ANCA) probes fluorescently discriminate between amyloid-β and prion plaques in brain. J Am Chem Soc. 2012 Oct 24;134(42):17338-41. PubMed.
- Bély M, Makovitzky J. Sensitivity and specificity of Congo red staining according to Romhányi. Comparison with Puchtler's or Bennhold's methods. Acta Histochem. 2006;108(3):175-80. PubMed.
- Janciauskiene S, Westin K, Grip O, Krakau T. Detection of Alzheimer peptides and chemokines in the aqueous humor. Eur J Ophthalmol. 2011 Jan-Feb;21(1):104-11. PubMed.
- Moncaster JA, Pineda R, Moir RD, Lu S, Burton MA, Ghosh JG, Ericsson M, Soscia SJ, Mocofanescu A, Folkerth RD, Robb RM, Kuszak JR, Clark JI, Tanzi RE, Hunter DG, Goldstein LE. Alzheimer's disease amyloid-beta links lens and brain pathology in Down syndrome. PLoS One. 2010;5(5):e10659. PubMed.
- Scinto LF, Daffner KR, Dressler D, Ransil BI, Rentz D, Weintraub S, Mesulam M, Potter H. A potential noninvasive neurobiological test for Alzheimer's disease. Science. 1994 Nov 11;266(5187):1051-4. PubMed.
- Scinto LF, Wu CK, Firla KM, Daffner KR, Saroff D, Geula C. Focal pathology in the Edinger-Westphal nucleus explains pupillary hypersensitivity in Alzheimer's disease. Acta Neuropathol. 1999 Jun;97(6):557-64. PubMed.
- Scinto LF, Frosch M, Wu CK, Daffner KR, Gedi N, Geula C. Selective cell loss in Edinger-Westphal in asymptomatic elders and Alzheimer's patients. Neurobiol Aging. 2001 Sep-Oct;22(5):729-36. PubMed.
- Scinto LF. ApoE allelic variability influences pupil response to cholinergic challenge and cognitive impairment. Genes Brain Behav. 2007 Apr;6(3):209-15. PubMed.
- Scinto LF. Pupillary cholinergic hypersensitivity predicts cognitive decline in community dwelling elders. Neurobiol Aging. 2008 Feb;29(2):222-30. PubMed.
- Litvan I, FitzGibbon EJ. Can tropicamide eye drop response differentiate patients with progressive supranuclear palsy and Alzheimer's disease from healthy control subjects?. Neurology. 1996 Nov;47(5):1324-6. PubMed.
- FitzSimon JS, Waring SC, Kokmen E, McLaren JW, Brubaker RF. Response of the pupil to tropicamide is not a reliable test for Alzheimer disease. Arch Neurol. 1997 Feb;54(2):155-9. PubMed.
- Iijima A, Haida M, Ishikawa N, Ueno A, Minamitani H, Shinohara Y. Re-evaluation of tropicamide in the pupillary response test for Alzheimer's disease. Neurobiol Aging. 2003 Oct;24(6):789-96. PubMed.
- Mahmoudian M, Ebrahimi SA, Kiani Z. An image processing technique for diagnosis of Alzheimer's disease. J Res Med Sci. 2009 Jul;14(4):205-9. PubMed.
Other Citations
External Citations
Further Reading
Primary Papers
- Michael R, Rosandić J, Montenegro GA, Lobato E, Tresserra F, Barraquer RI, Vrensen GF. Absence of beta-amyloid in cortical cataracts of donors with and without Alzheimer's disease. Exp Eye Res. 2013 Jan;106:5-13. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Banner Alzheimer's Institute
There appear to be major differences in the quality of the stained images between the two papers, suggesting perhaps that the reasons lie in the particular staining technique used (Bennhold's technique used by Goldstein et al. and Moncaster et al. vs. Puchtler's technique used by Michael et al., who also used a different immunostaining antibody).
This raises the following question: If the goal was to replicate the results of Goldstein et al., 2003, in AD, and Moncaster et al., 2010, in Down's syndrome, does it perhaps make sense to use the identical staining technique? It may be worth noting that Frederikse, 2000, was also successful in immunostaining and Congo red staining of Aβ in human lenses. There is also a chance that differences in postmortem interval and in handling specimens could have contributed to the observed differences in results.
From my perspective as a clinician, the upshot is that there are potentially crucial technical differences among the studies in question. However, there appear to be sufficient in-vitro data to justify in-vivo studies in humans. Our site participates in the ongoing Cognoptix study; I have also served as a consultant to them on study design. I also note that Cognoptix announced that, by detecting a specific fluorescent signature of ligand-marked β amyloid in the supranucleus region of the human lens, their so-called SAPPHIRE II system showed a twofold differentiation factor between small groups of five healthy volunteers and five patients diagnosed with probable Alzheimer s disease in a recent proof-of-concept clinical trial. I do not believe that these data have been published.
References:
Frederikse PH. Amyloid-like protein structure in mammalian ocular lenses. Curr Eye Res. 2000 Jun;20(6):462-8. PubMed.
Make a Comment
To make a comment you must login or register.