Nicastrin—A DAPper Role in γ-Secretase
Quick Links
Though we've known for some time now that the protein nicastrin is a major player in the γ-secretase complex (see ARF related news story), its exact role has remained somewhat elusive. But in last Friday's Cell, Gang Yu, Thomas Sudhof, and colleagues at the University of Texas Southwestern Medical Center, Dallas, report quite a dapper role for nicastrin—it introduces Notch and the Aβ precursor protein (AβPP) to the presenilins and it does so by virtue of its DAP domain. The finding not only helps resolve the burning question of how γ-secretase recognizes its substrates, but it could also open up new therapeutic strategies for preventing production of amyloid-β (Aβ).
Yu had previously shown that nicastrin can bind to γ-secretase substrates (see Yu et al., 2000). To get a better picture of how this happens, first author Sanjiv Shah and colleagues focused on various parts of the transmembrane protein. Using immunoprecipitation experiments, they found that the N-terminal part of nicastrin interacts with C99, the γ-secretase substrate that remains after AβPP's ectodomain is shed through β-secretase cleavage. More specifically, Shah and colleagues found that it is nicastrin's ectodomain that binds to C99 and also to N100, the analogous piece of the signaling molecule Notch.
Shah and colleagues next focused on a portion of nicastrin's ectodomain, the DAP domain, so called because it harbors two highly conserved motifs—a DYIGS amino acid sequence found in nicastrin and its orthologs and a peptidase homologous region. The authors found that a 28-amino-acid region in this DAP domain is needed for γ-secretase activity. Using nicastrin mutants, they also found that one specific amino acid, glutamate 333 (E333), is essential for activity. Replacing this with alanine, arginine, serine, or another dissimilar amino acid abolished activity, whereas conservative replacement with an aspartate had little effect.
Of course, there are many ways to inhibit γ-secretase activity, one being to prevent formation of the core complex, which comprises presenilin (1 or 2), Aph1, Pen2, and nicastrin. However, Shah and colleagues found that while mutating the transmembrane region of nicastrin abolished endoproteolysis of presenilin, a prerequisite for maturity of the complex, E333A mutations had no effect on complex maturation. The results suggest that the DAP domain is not involved in the formation of the complex, but is required for the complex to function, once formed. "These data agree with a model in which assembly of nicastrin, APH-1, presenilin, and PEN-2 precedes presenilin endoproteolysis, which subsequently is required for activation of γ-secretase activity to cleave type I membrane proteins. Moreover, these results provide compelling evidence that [the] nicastrin DAP domain plays a direct functional role in γ-secretase-substrate recognition," write the authors. In support of this, they found that a short peptide that mimics the γ-secretase cleavage site is not cleaved by recombinant γ-secretase made using nicastrin mutated in the transmembrane domain, which fits with the essential role of this region in complex assembly. In contrast, recombinant secretase made with the E333A mutant of nicastrin did cleave the peptide mimic, bolstering the idea that the ectodomain of nicastrin is involved in recognizing larger γ-secretase substrates (see figure).
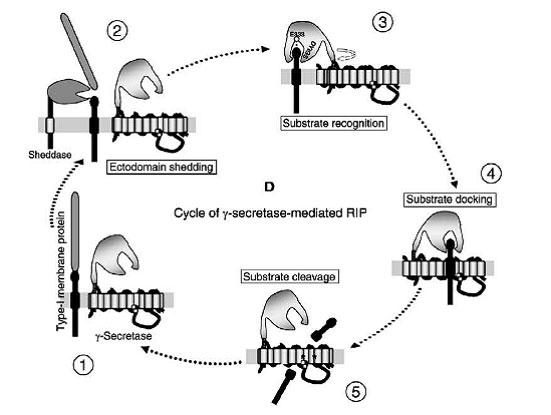
Nicastrin’s Role Recognized
The authors propose a model for regulated intramembrane cleavage (RIP) whereby after ectodomain shedding (2), nicastrin recognizes the remaining stubs on the outer surface of the membrane (3) and then docks them with the catalytic core of γ-secretase (4), setting up the substrates for cleavage (5). [Image courtesy of Cell Press]
While these results shed more light on the role of nicastrin and the functioning of γ secretase, they also might lead to novel therapeutics. Shah and colleagues found, for example, that a free amino terminus is a prerequisite for cleavage by γ-secretase. When the authors blocked the N-terminal of C99 with an antibody, proteolysis was prevented. While an antibody obviously adds considerable bulk to the N-terminus of C99, the much smaller biotin and fluorescein moieties also blocked cleavage when attached to the N-terminus, raising the possibility that small molecules that bind to the extracellular head of C99 could prevent formation of Aβ.—Tom Fagan
References
News Citations
Paper Citations
- Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang DS, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C, Rogaev E, Smith M, Janus C, Zhang Y, Aebersold R, Farrer LS, Sorbi S, Bruni A, Fraser P, St George-Hyslop P. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature. 2000 Sep 7;407(6800):48-54. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, Südhof T, Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005 Aug 12;122(3):435-47. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Kansas
This is an excellent study from the lab of Gang Yu, the original discoverer of nicastrin as a presenilin partner needed for γ-secretase activity when he was with Peter St. George-Hyslop in Toronto. In his new lab at UT Southwestern, Yu has nailed down an essential role for nicastrin in substrate recognition. To date, virtually nothing has been known about the specific biochemical role of nicastrin in γ-secretase activity. Nicastrin (NCT) is needed for assembly and maturation of the protease complex, including presenilin endoproteolysis into N-terminal fragment (NTF) and C-terminal fragment (CTF) subunits, and the NCT transmembrane domain is critical for these events. However, all the action (substrate binding and catalysis) has so far appeared to be taking place on presenilin. Recent work from our lab (Kornilova et al., 2005) located a substrate docking site at the interface between the two presenilin subunits, at a site distinct from the active site, which is also located at the NTF/CTF interface. The implication is that substrate docks and then squeezes between the presenilin subunits to access the internal active site, which contains water and the two catalytic aspartates (also see ARF related news story. We suggested that presenilin is the γ-secretase component that directly interacts with the substrate transmembrane domain, and nothing in the new study from the Yu lab contradicts this idea. However, we also suggested that the primary role of nicastrin, along with Aph-1 and Pen-2, is to render PS competent for proteolysis, but Yu and his colleague have clearly demonstrated that nicastrin also plays an essential role in substrate binding, specifically interacting with the substrate N-terminus.
In the new study, the evidence for a role in substrate recognition is overwhelming. First, the nicastrin ectodomain interacts directly with APP- and Notch-based membrane stubs, and expression of the nicastrin ectodomain alone interferes with γ-secretase cleavage of these substrates. Second, an aminopeptidase-like (AP) region in nicastrin is essential for interaction with substrate. This region lacks residues that would be needed for peptidase activity but apparently still retains residues (e.g., a critical aspartate) capable of substrate binding. Consistent with the requirement for the AP region, blocking the amino-terminus of the substrate in a variety of ways prevented interaction with nicastrin and γ-secretase proteolysis. Even N-formylation, a very subtle structural change, rendered the substrate uncleavable. Thus, it is clear that the nicastrin ectodomain directly interacts with the N-terminus of substrates and that this interaction is essential for proteolysis. It is interesting to note, however, that a small fluorogenic peptide was proteolyzed by AP-mutant nicastrin the same as wild-type, demonstrating that the mutant complex is still a fully active protease. Nevertheless, endogenous substrates, with their N-termini sticking out of the membrane, must pass the nicastrin gatekeeper.
Nicastrin may be a gatekeeper, but the order of substrate binding events is unclear: Although Yu and colleagues suggest a model where the nicastrin-substrate interaction leads to substrate docking on presenilin, substrate binding to nicastrin and presenilin may be virtually simultaneous or even in the reverse order (presenilin first, nicastrin second). In the context of the full γ-secretase complex, substrate may be inaccessible to the AP domain of nicastrin until docking on presenilin. In this scenario, nicastrin's role would be to draw the substrate further into the complex and help shepherd it from docking site to active site. However, these are matters for future study and do not take anything away from what is a very important advance in our understanding of γ-secretase.
References:
Kornilova AY, Bihel F, Das C, Wolfe MS. The initial substrate-binding site of gamma-secretase is located on presenilin near the active site. Proc Natl Acad Sci U S A. 2005 Mar 1;102(9):3230-5. PubMed.
UK Dementia Research Institute@UCL and VIB@KuLeuven
This is an excellent paper.
Yale University School of Medicine
The strength of this paper is the imaginative use of carefully crafted recombinant peptides to study a complicated process that is difficult to approach using conventional cell biological techniques. But one also has to keep in mind that no "native" molecules are actually studied under in vivo conditions. Recombinant C99 peptides are often studied as a more accessible form of the amyloid-β precursor protein (AβPP), the physiological substrate of γ-secretase, and when they are expressed in living cells, one can be reasonably confident that they are mimicking the endogenous molecules. But a consideration in this study is whether they also behave the same way in detergent extracts. Following cleavage by β-secretases, the natural C99 peptides are likely to be dimers in situ, possibly sequestered in lipid raft-like domains, and still attached to an elaborate cytoskeletal network in the adjacent cytoplasm. We have no idea how recombinant-derived peptides are arranged in detergent extracts. If they exist as small micelles, as is likely, are they sticky? Do they bind other proteins that are present in the various cell extracts, such as APP or a presenilin, or some active factor yet unknown? One would also like to know the size and properties of the C99-nicastrin complexes; their behavior on sucrose gradients and by gel filtration might be informative, as well as some idea of the strength of their interactions.
The functional assays involving cleavage activities with various recombinant substrates are revealing, but the activities seem to be very low, requiring many hour-long incubations at 37 degrees. The use of cells derived from nicastrin (Nct) knockouts to assay the Nct mutants is another potential complication. They are described as being from embryos that lack γ-secretase, yet they have undergone some development presumably without Notch activation. Are there compensating mechanisms operating in these cells? The claim that the amino terminal amino acid of C99 is critical for Nct recognition needs more experimental support. Can this interaction be blocked by small synthetic peptides derived from the amino terminus of C99?
One has to raise these questions, since similar studies from the Wolfe lab also provide a convincing case that substrate binding and catalysis of C99 and C83 take place at specific sites on presenilin, with nicastrin relegated to a supporting role.
German Center for Neurodegenerative Diseases (DZNE)
It has been known for a while that the ectodomain of type I membrane proteins needs to be proteolytically trimmed before the proteins can be further processed by γ-secretase. This led to the speculation that γ-secretase—and more specifically, the nicastrin subunit within the complex—must somehow be able to act as a molecular ruler and measure the length of the ectodomain of the γ-secretase substrates. Just how this can happen remained unclear. The elegant and convincing work by Shah and colleagues shows that, in fact, nicastrin can act as the molecular ruler. Their appealing model proposes that nicastrin sticks out of the membrane like a crane, binds the substrate, and shifts it to its docking or processing site in the γ-secretase complex. The free N-terminus of a substrate protein seems to be the primary determinant for recognition. Thus, nicastrin should be able to bind substrates regardless of their primary sequence—as long as they have a short ectodomain and contain a transmembrane domain. This model fits well with previous data by us and others, showing that γ-secretase has a relaxed sequence specificity. In future studies, it will be exciting to study in molecular detail how the γ-substrate can move (or be pushed) for proteolytic processing into the active site of the γ-secretase complex.
bio-chemistry-psychology/neuroscience graduate.
I find this article very informative, however, it is merely educational. While I find it interesting, it is not by finding every molecular binding site and protein involved in AD pathogenesis that we will get us closer to a cure. I love molecular and cellular mechanisms of life... in the words of the great pilot D.P. Davies: "let's get on with it."
�
Excellent, and a very useful study by Yu et al. The article has proved to be quite informative to me, and I am sure will assist me in my research work. Thank you for this commendable work.
Make a Comment
To make a comment you must login or register.