More Evidence that γ-Secretase Modulators Spare Essential Substrates
Quick Links
With recent trial failures dashing hopes for treating Alzheimer’s with γ-secretase inhibitors (see ARF news story and ARF news story), scientists are redoubling efforts to develop compounds that tweak this enzyme’s activity rather than stymie it altogether. γ-secretase modulators, as they are called, shift where the enzyme trims amyloid precursor protein (APP) so as to generate less Aβ42 while preserving total output of Aβ and APP’s C-terminal fragment. An August 2 Nature Communications paper lends further credence to this strategy. Using mass spectrometry and biochemical approaches, researchers led by Patrick Fraering of the Swiss Federal Institute of Technology, Lausanne, Switzerland, showed that γ-secretase modulators curb cleavage of APP and another substrate, Notch, at the so-called γ site, without stifling production of intracellular signaling molecules derived from ε-site cleavage, which occurs a few amino acids closer to the C-terminus of these substrates. The work supports the contention that γ-secretase modulators “do not have the harmful side effects caused by γ-secretase inhibitors,” Fraering told Alzforum. In contrast, the scientists reported that several APP mutations linked to familial AD (FAD) enhance cleavage at both γ- and ε-sites, yet the γ-secretase modulators still worked in these FAD mutants, suggesting the compounds may succeed in people with familial as well as sporadic forms of AD.
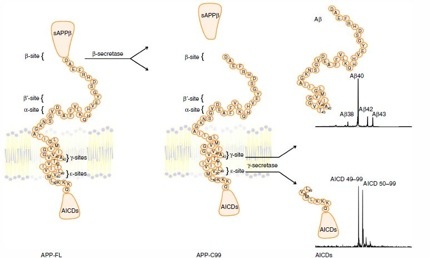
According to the sequential processing model of intramembrane proteolysis, γ-secretase slices the transmembrane portion of APP first at the ε-sites yielding APP intracellular domain (AICD) 49-99 and AICD 50-99, then at the γ-sites to generate Aβ peptides— typically more Aβ40 than the deadlier Aβ42. Gamma-secretase processing of Notch occurs in similar fashion, and research blames loss of intracellular Notch fragments for the gastrointestinal and skin defects linked to γ-secretase inhibitors (see model below).
Sequential intramembrane proteolysis
Image courtesy of Nature and Patrick Fraering [Click image to enlarge.]
In the current study, lead author Mitko Dimitrov and colleagues analyzed γ-secretase processing of APP or Notch in the absence or presence of γ-secretase modulators (GSMs). They purified active γ-secretase from mammalian cells (see Alattia et al., 2013), mixed in APP or Notch tagged with the Flag peptide for tracking, and added either a control solution or one of two second-generation GSMs. One GSM was a non-steroidal anti-inflammatory drug (NSAID)-like compound (GSM-1) developed by Merck. GSM-1 binds γ-secretase allosterically (see ARF news story) and improves cognition in AD transgenic mice (Mitani et al., 2012). The other GSM was a non-NSAID-like molecule (E2012) developed at Eisai. Neither is in clinical development at present, though the Eisai compound had previously reached Phase 1 before eye problems in the treated group stopped that trial (see ARF conference story)
Mass spectrometry of the reaction products showed that both GSMs bumped up Aβ38 levels and lowered Aβ42, yet left intracellular ε-cleavage fragments unchanged for APP and Notch. Though prior studies had already suggested that GSMs spare ε-site processing (see McKee et al., 2013; ARF related news story), the mass spectrometry data was “a nice way to show it unequivocally and with detail that was not present in the other papers,” said Barbara Tate, a biotech consultant in Cambridge, Massachusetts. Tate was formerly vice president of Satori Pharmaceuticals, which had been developing γ-secretase modulators for AD before closing down in June (see ARF news story).
The present study also revisits an older theory associating neurotoxicity with intracellular APP fragments, and Fraering said this could explain what makes some FAD mutations in APP especially dangerous. In the cell-free γ-secretase assay, APP with transmembrane FAD mutations T714I and V715A not only increased Aβ38/40 and Aβ42/40 ratios relative to wild-type APP, consistent with previous work (Chávez-Gutiérrez et al., 2012), but mass spectrometry revealed skewed ε-processing that heavily favors the AICD 49-99 fragment. “AICD 50-99 is totally gone,” Fraering said. This indicates that transmembrane FAD mutations in APP affect not only γ- but also the ε-sites. “The combination of higher Aβ42/40 ratio and the loss of AICD 50-99 can explain the extreme aggressiveness of the [T714I and V715A] APP mutations with regard to the onset of the disease,” the authors write. Prior mouse studies have implicated expression of C-terminal APP fragments in brain amyloidosis and neurodegeneration (see Kammescheidt et al., 1992; Oster-Granite et al., 1996).
Finally, to learn more about how GSMs work at a molecular level, Dimitrov and colleagues used computer-based simulations to model how FAD mutations affect the structure of APP in lipid membranes. They found that the mutations tilted APP’s orientation in the membrane in a manner that leaves the Aβ38 cleavage site more exposed. This provides a potential explanation for the elevated Aβ38 levels associated with the T714I and V715A APP mutants, Fraering said. "It remains largely unknown whether higher Aβ38 is protective," he told Alzforum. "To the best of my knowledge, nobody has ever demonstrated that Aβ38 is protective. Only that reduced Aβ42 is"
All told, the new data “support that GSMs are a viable therapeutic approach,” Tate said. “They are more like lasers than sledgehammers.” However, she said, the molecular modeling suggests that drug developers will continue to struggle, since critical APP domains “are embedded in the membrane in a way that makes them difficult for small molecules to reach.”
Yasuyuki Mitani of Astellas Pharma Inc., Tsukuba, Japan, was intrigued by the possibility that impaired intracellular APP processing could explain why some FAD mutants are especially harmful. However, he worries that the cell-free assay with exogenous APP is artificial and wonders if the findings can be reproduced in cellular or in vivo studies. Steve Wagner of the University of California, San Diego, expressed similar concern, noting that the V717F APP mutation did not increase the Aβ42/40 ratio in the present study. That was “a big red flag,” he said, because it disagrees with previous work showing that mutations at this site do drive up relative Aβ42 levels in cells (Suzuki et al., 1994), AD transgenic mice (Esh et al., 2005), and patient fibroblasts (Scheuner et al., 1996). The current findings “need to be extended to a more physiologically relevant system,” Wagner said.
Several companies are developing γ-secretase modulators (see ARF news story), though only a few have purportedly entered clinical testing. After Bristol Myers Squibb halted development of its γ-secretase inhibitor in Phase 2 due to cognitive worsening in treated participants, the company announced in late 2012 that it has an “investigational gamma-secretase modulator in Phase 1 development.” Clinicaltrials.gov lists a Phase 1 trial measuring safety and tolerability of Eisai’s oral gamma-secretase modulator (E2212) in healthy adults.—Esther Landhuis
References
News Citations
- Lilly Halts IDENTITY Trials as Patients Worsen on Secretase Inhibitor
- Drug Company Halts Development of γ-Secretase Inhibitor Avagacestat
- Evidence Mounts That Some γ-Secretase Modulators Bind Presenilin
- Barcelona: Allosteric γ Modulation Moves Toward Clinic
- New γ-Secretase Modulators Reduce Aβ42, Avoid Notch
- Satori Pharmaceuticals Shuts Down, Abandons γ-Secretase Modulators
Paper Citations
- Alattia JR, Matasci M, Dimitrov M, Aeschbach L, Balasubramanian S, Hacker DL, Wurm FM, Fraering PC. Highly efficient production of the Alzheimer's γ-Secretase integral membrane protease complex by a multi-gene stable integration approach. Biotechnol Bioeng. 2013 Jan 28; PubMed.
- Mitani Y, Yarimizu J, Saita K, Uchino H, Akashiba H, Shitaka Y, Ni K, Matsuoka N. Differential effects between γ-secretase inhibitors and modulators on cognitive function in amyloid precursor protein-transgenic and nontransgenic mice. J Neurosci. 2012 Feb 8;32(6):2037-50. PubMed.
- McKee TD, Loureiro RM, Dumin JA, Zarayskiy V, Tate B. An improved cell-based method for determining the γ-secretase enzyme activity against both Notch and APP substrates. J Neurosci Methods. 2013 Feb 15;213(1):14-21. PubMed.
- Chávez-Gutiérrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, Lismont S, Zhou L, Van Cleynenbreugel S, Esselmann H, Wiltfang J, Serneels L, Karran E, Gijsen H, Schymkowitz J, Rousseau F, Broersen K, De Strooper B. The mechanism of γ-Secretase dysfunction in familial Alzheimer disease. EMBO J. 2012 May 16;31(10):2261-74. Epub 2012 Apr 13 PubMed.
- Kammesheidt A, Boyce FM, Spanoyannis AF, Cummings BJ, Ortegón M, Cotman C, Vaught JL, Neve RL. Deposition of beta/A4 immunoreactivity and neuronal pathology in transgenic mice expressing the carboxyl-terminal fragment of the Alzheimer amyloid precursor in the brain. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10857-61. PubMed.
- Oster-Granite ML, McPhie DL, Greenan J, Neve RL. Age-dependent neuronal and synaptic degeneration in mice transgenic for the C terminus of the amyloid precursor protein. J Neurosci. 1996 Nov 1;16(21):6732-41. PubMed.
- Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994 May 27;264(5163):1336-40. PubMed.
- Esh C, Patton L, Kalback W, Kokjohn TA, Lopez J, Brune D, Newell AJ, Beach T, Schenk D, Games D, Paul S, Bales K, Ghetti B, Castaño EM, Roher AE. Altered APP processing in PDAPP (Val717 --> Phe) transgenic mice yields extended-length Abeta peptides. Biochemistry. 2005 Oct 25;44(42):13807-19. PubMed.
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996 Aug;2(8):864-70. PubMed.
Other Citations
External Citations
Further Reading
Papers
- Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, Ahn K, Nguyen P, Comer D, Mao L, Yu C, Pleynet D, Digregorio PJ, Velicelebi G, Stauderman KA, Comer WT, Mobley WC, Li YM, Sisodia SS, Tanzi RE, Wagner SL. Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Neuron. 2010 Sep 9;67(5):769-80. PubMed.
- Mitani Y, Yarimizu J, Saita K, Uchino H, Akashiba H, Shitaka Y, Ni K, Matsuoka N. Differential effects between γ-secretase inhibitors and modulators on cognitive function in amyloid precursor protein-transgenic and nontransgenic mice. J Neurosci. 2012 Feb 8;32(6):2037-50. PubMed.
News
- Barcelona: Live and Learn—γ-Secretase Inhibitors Fade, Modulators Rise
- Paris: Semagacestat Autopsy and Other News of Trial Tribulations
- Paper Alert: γ-Secretase Modulators Trump Inhibitors
- Satori Pharmaceuticals Shuts Down, Abandons γ-Secretase Modulators
- New γ-Secretase Modulators Reduce Aβ42, Avoid Notch
Primary Papers
- Dimitrov M, Alattia JR, Lemmin T, Lehal R, Fligier A, Houacine J, Hussain I, Radtke F, Dal Peraro M, Beher D, Fraering PC. Alzheimer's disease mutations in APP but not γ-secretase modulators affect epsilon-cleavage-dependent AICD production. Nat Commun. 2013;4:2246. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
�
The finding of a decrease in the AICD 49-99/50-99 ratio due to APP mutations near the γ- or ε-site is intriguing, and may partly answer why these mutations increase the Aβ 42/40 ratio if the step-wise cleavage hypothesis is correct.
That GSMs have no effect on the epsilon cleavage is a common knowledge. GSM-1 has been proposed to bind to presenilin rather than APP, resulting in the conformation change in the pore of γ -secretase, which may explain the present finding that GSM-1 works regardless of having FAD mutations in APP. I am curious whether these findings will be reproduced in the cellular or even in vivo studies because a cell-free system with exogenous APP is somewhat artificial and sometimes shows different phenotypes from that observed in the cellular system.
View all comments by Yasuyuki MitaniMake a Comment
To make a comment you must login or register.