The Guardians of Forever: Forming and Keeping Memories
Quick Links
Older adults can still remember some of their childhood as though it happened yesterday. What keeps these memories around for a lifetime? This question has been shrouded in mystery, but scientists began to draw back the veil in 2006 by identifying a molecule in rats that maintains old memories. Now, researchers led by Yadin Dudai at the Weizmann Institute of Science in Rehovot, Israel, and Todd Sacktor at SUNY Downstate Medical Center, Brooklyn, New York, have taken this research a step further by showing that overexpression of the molecule, protein kinase Mζ (PKMζ), can strengthen memories long after they have formed. The research, reported in today’s Science, solidifies the case for the kinase having a unique role as a guardian of memory.
“This is an important paper,” said Oliver Hardt at McGill University, Montréal, Canada, who was not involved in the work. The data might inspire some scientists in the field to refocus their energy, Hardt suggested, shifting from work on memory formation and consolidation to a greater interest in long-term memory maintenance.
This week saw another advance in memory research as well, as a paper in today’s Cell describes an essential role for astrocytes in long-term memory formation. Researchers led by Cristina Alberini at Mount Sinai School of Medicine in New York City, and Pierre Magistretti at the University of Lausanne, Switzerland, found that lasting memories did not form unless astrocytes metabolized glycogen stores and released lactate, which was then taken up by neurons. The scientists showed that lactate was essential to stabilize long-term potentiation of synapses and trigger many of the downstream molecules that help build memories in neurons, although the mechanism remains mysterious.
Dudai and Sacktor’s work follows five years after the first discovery of PKMζ’s unexpected abilities. Sacktor and André Fenton at New York University showed that by administering an inhibitor of PKMζ in the insular cortex of rats, they could erase a conditioned taste aversion that had been learned three months earlier (see ARF related news story on Pastalkova et al., 2006). This was significant because PKMζ, an isoform of protein kinase C, is, to date, the only molecule that has been shown to sustain long-term, consolidated memories, scientists contacted for this story agreed. “Identification of PKMζ was a pretty big event,” Hardt said. “It was the molecule everyone had been looking for.” Science magazine hailed the finding as one of its 10 “Breakthroughs of the Year 2006.” Later work in the Sacktor lab showed that PKMζ not only acted in the insular cortex, but also in the hippocampus and neocortex (see Shema et al., 2007 and Shema et al., 2009), suggesting it is broadly active in the brain. One of the corollaries of the initial finding, Fenton said, was that “if PKMζ is really storing memories, then in principle, increasing PKMζ expression should improve memories.”
Dudai, Sacktor, and colleagues tested this hypothesis by designing lentiviral vectors to express either PKMζ or a dominant-negative version of the kinase. First author Reut Shema microinfused the viruses into the insular cortex of rats six days after he trained the animals to develop a dislike of a new taste by pairing it with lithium chloride, which makes rats sick. He chose this time period because by six days after training, memory is fully consolidated in rats (see Rosenblum et al., 1993). When the animals were tested seven days after viral infusion, the dominant-negative molecule had erased the learned aversion, just as the PKMζ inhibitor did in earlier experiments.
Taste aversion is normally a very strong memory, incapable of showing further strengthening, so for the trials of functioning PKMζ, Shema and colleagues produced a weaker version of the memory by adding much lower concentrations of lithium chloride to the new taste. The scientists found that administering PKMζ either a week before or a week after taste training significantly strengthened this weak memory. Not only that, but the more PKMζ that was expressed in the cortex, the stronger the memory became, showing a direct relationship between the kinase and memory intensity.
“This research adds, in a very significant and different way, to the evidence that PKMζ is a mechanism for storing memories,” Fenton concludes. He was not involved in the current paper. The burning question now is how the kinase produces these effects. Recent research has shown that PKMζ speeds up the transport of GluR2 subunits of AMPA receptors, Fenton said, probably by phosphorylating the trafficking machinery (see Migues et al., 2010). The upshot is that when PKMζ is present at a synapse, AMPA receptors are inserted at twice the normal rate, Fenton said. This results in twice as many AMPA receptors at the synapse, more uptake of glutamate, and stronger synaptic responses. When the kinase is inhibited, Fenton said, the AMPA insertion rate falls, and endocytosis catches up and trims the synapse strength back to baseline levels.
The model suggests that PKMζ should act only on synapses that are somehow “tagged” as part of a memory. Some research does show that the kinase accumulates preferentially in tagged synapses, where it maintains their long-term potentiation (see Sajikumar et al., 2005). In their overexpression experiments, Dudai and colleagues saw that only some dendritic spines contained PKMζ (see image below), lending further support to the idea that PKMζ is specifically sorted to tagged synapses. In ongoing work, Dudai said, he is seeking to test this hypothesis and discover how such a targeting mechanism might work. Dudai would also like to identify which subclasses of neurons are most affected by PKMζ overexpression, by using viruses that target gene expression into specific neuron types. Kurt Haas at the University of British Columbia in Vancouver notes that this model implies that PKMζ would have to remain in tagged synapses for the lifetime of a memory. “It is a fascinating shift in paradigms of thinking about how memories are stored,” he said.
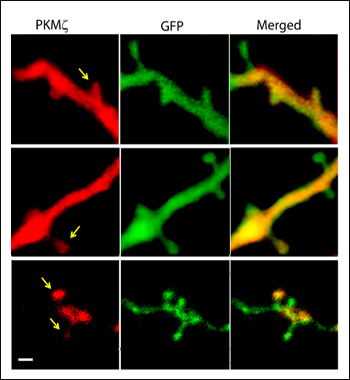
Protein kinase Mζ (red) accumulates in some dendritic spines (yellow), but not in others (green). This supports the idea that PKMζ specifically targets synapses that have been marked for memory storage, although the hypothesis remains to be proven. Image credit: Weizmann Institute of Science, Rehovot, Israel
Dudai said that he has no current plans to test his PKMζ vectors in Alzheimer’s disease mouse models, because his immediate focus is to understand how the kinase works. Nonetheless, he believes that the protein might have an important role in cognitive disorders, and that this could be a fruitful line of research. Hardt suggests that the PKMζ findings might help shift scientists’ focus from treating the pathological symptoms of memory disorders to repairing or improving the machinery that maintains memory. Fenton notes that PKMζ and GluR2 subunits have been shown to build up in neurofibrillary tangles (see Crary et al., 2006)—an intriguing finding that implies PKMζ trafficking could be perturbed in AD and other dementias. The unknown mechanism that causes tangles to soak up the kinase might make a promising therapeutic target, Fenton suggested. Nonetheless, Fenton cautions it is too early to consider PKMζ overexpression itself as a treatment approach for cognitive disorders, because there is still so much basic research to be done before scientists understand what this molecule is doing.
One of the central problems for developing therapies, Fenton said, is that researchers still do not understand what memory is at a molecular level. Hardt takes it a step further, pointing out that not only do scientists not understand what memory is, but they know even less about forgetting. "What does a synapse look like before and after forgetting?" Hardt asked. Is a forgotten memory irretrievable? No one knows, but perhaps further study of PKMζ will shed light on this mystery.
In the Cell paper, Alberini, Magistretti, and colleagues took quite a different approach to the problem of memory, examining the role played by astrocytes. These glial cells, which outnumber neurons, were once considered mere second-string support players to the neuronal stars. In recent years, however, research has shown that astrocytes are much more essential to neurons than was thought, for example, having crucial roles in promoting synapse formation and elimination (see ARF related news story on Christopherson et al., 2005 and ARF related news story on Stevens et al., 2007).
Astrocytes are also metabolically coupled to neurons. These glia can break down glycogen energy stores, while neurons cannot. It is believed that neurons soak up the lactate released by astrocytes and use it as fuel. Neuronal activity stimulates astrocytes in turn, leading them to break down glucose (see Pellerin and Magistretti, 1994) and produce lactate (see Fray et al., 1996; Urrila et al., 2004).
To find out if this metabolic crosstalk was crucial to memory formation, first author Akinobu Suzuki began by confirming that lactate levels rose in the hippocampus of rats after they were trained to avoid a foot shock. When Suzuki and colleagues pharmacologically inhibited glycogen breakdown either immediately before or after training, lactate levels did not rise, as might be expected. This condition wiped out the rats’ long-term memory, although their short-term memory remained fine. The researchers rescued memory formation by injecting lactate into the hippocampus before training. The results suggested that lactate release is, in fact, essential for long-term memory formation.
At a mechanistic level, Suzuki and colleagues found that when glycogen breakdown was inhibited, long-term potentiation (LTP), a form of synaptic strengthening that is essential for learning, initially developed but did not last. Lactate injections rescued LTP. The authors also knocked down the levels of the lactate transporters MCT1, MCT2, and MCT4 using antisense oligonucleotides. For MCT1 and MCT4, reported to be enriched in astrocytes (see Pellerin et al., 2005), knockdown disrupted long-term memory, while lactate injection rescued it. Knockdown of MCT2, found in neurons, eliminated memory but lactate did not rescue it. The results fit with a model in which lactate must be transported out of astrocytes and taken up by neurons for learning to occur. Suzuki and colleagues also showed that MCT1 expression increases in response to learning; when they knocked down MCT1, long-term memories did not form. Finally, memory consolidation is known to require changes in numerous neuronal molecules, such as induction of activity-regulated cytoskeletal protein and phosphorylation of the transcription factor CREB and of cofilin, which is involved in remodeling the cytoskeleton. The authors showed that these changes were also blocked by glycogenolysis inhibition and rescued by lactate.
The million-dollar question now, Alberini said, is, What is lactate doing in neurons? It is possible that lactate is simply providing energy. However, the authors found that glucose injections only weakly and transiently rescued memory formation, suggesting that merely supplying an energy source is not enough to produce these effects. Alberini said the next step will be to determine how lactate is acting on neurons. She is also interested in examining animal models of cognitive impairment for defects in lactate production. Additionally, Alberini would like to look at brains from AD patients, measuring the expression of lactate transporters and astrocytic markers to see if this metabolism is disturbed.
Dmitri Rusakov at University College London, U.K., wrote to ARF that the identification of lactate’s role in memory formation is exciting. The findings also suggest that adding lactate could rescue memory impairment caused by faulty glycogenolysis, he wrote, but it remains to be seen how common this mechanism is in memory disorders (see full comment below).
Ben Barres at Stanford University in Palo Alto, California, noted in an e-mail that recent gene expression data from large array studies of purified cell populations have cast doubts on the older lactate transporter expression data (see Cahoy et al., 2008 ; Doyle et al., 2008; Dougherty et al., 2010). For example, the newer data indicate that MCT4 is not expressed in astrocytes, and MCT2 is not exclusive to neurons. Noting also that antisense knockdown does not eliminate expression, Barres suggested that the next step should be to use more specific molecular perturbations in defined cell populations to nail down the contributions of the different transporters.
For her part, Alberini said the most important thing is for researchers to enlarge their frame of thinking to include not just neurons but other cells as well. “Before, we were thinking about cognitive functions as a result of neuronal network mechanisms, but we now have to think about the crosstalk between astrocytes and neurons,” she said.—Madolyn Bowman Rogers
References
News Citations
- Immune Receptor Controls Synaptic Plasticity; LTP Makes Memories
- Perinatal Soup—Early Pathogen or Toxin Exposures Leave Brain Vulnerable
- Paper Alert: Does the Complement Devour Synapses?
Paper Citations
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006 Aug 25;313(5790):1141-4. PubMed.
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007 Aug 17;317(5840):951-3. PubMed.
- Shema R, Hazvi S, Sacktor TC, Dudai Y. Boundary conditions for the maintenance of memory by PKMzeta in neocortex. Learn Mem. 2009 Feb;16(2):122-8. PubMed.
- Rosenblum K, Meiri N, Dudai Y. Taste memory: the role of protein synthesis in gustatory cortex. Behav Neural Biol. 1993 Jan;59(1):49-56. PubMed.
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010 May;13(5):630-4. PubMed.
- Sajikumar S, Navakkode S, Sacktor TC, Frey JU. Synaptic tagging and cross-tagging: the role of protein kinase Mzeta in maintaining long-term potentiation but not long-term depression. J Neurosci. 2005 Jun 15;25(24):5750-6. PubMed.
- Crary JF, Shao CY, Mirra SS, Hernandez AI, Sacktor TC. Atypical protein kinase C in neurodegenerative disease I: PKMzeta aggregates with limbic neurofibrillary tangles and AMPA receptors in Alzheimer disease. J Neuropathol Exp Neurol. 2006 Apr;65(4):319-26. PubMed.
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005 Feb 11;120(3):421-33. PubMed.
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007 Dec 14;131(6):1164-78. PubMed.
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10625-9. PubMed.
- Fray AE, Forsyth RJ, Boutelle MG, Fillenz M. The mechanisms controlling physiologically stimulated changes in rat brain glucose and lactate: a microdialysis study. J Physiol. 1996 Oct 1;496 ( Pt 1):49-57. PubMed.
- Urrila AS, Hakkarainen A, Heikkinen S, Vuori K, Stenberg D, Häkkinen AM, Lundbom N, Porkka-Heiskanen T. Stimulus-induced brain lactate: effects of aging and prolonged wakefulness. J Sleep Res. 2004 Jun;13(2):111-9. PubMed.
- Pellerin L, Bergersen LH, Halestrap AP, Pierre K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res. 2005 Jan 1-15;79(1-2):55-64. PubMed.
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008 Jan 2;28(1):264-78. PubMed.
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, Gong S, Greengard P, Heintz N. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008 Nov 14;135(4):749-62. PubMed.
- Dougherty JD, Schmidt EF, Nakajima M, Heintz N. Analytical approaches to RNA profiling data for the identification of genes enriched in specific cells. Nucleic Acids Res. 2010 Jul;38(13):4218-30. PubMed.
Further Reading
Primary Papers
- Shema R, Haramati S, Ron S, Hazvi S, Chen A, Sacktor TC, Dudai Y. Enhancement of consolidated long-term memory by overexpression of protein kinase Mzeta in the neocortex. Science. 2011 Mar 4;331(6021):1207-10. PubMed.
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011 Mar 4;144(5):810-23. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Blanchette Rockefeller Neurosciences Institute
These interesting results are consistent with a large body of previous research showing that PKC is involved in associative learning and memory. Much of the previous research dating back to the 1980s focused on conventional and novel PKC isoforms, showing that PKCα and ε are activated and translocated to dendritic membranes after associative learning (1-3). PKC activators have also been found to have therapeutic benefits for Alzheimer's disease transgenic mice, reducing Aβ and increasing the number of mushroom spine synapses (4).
PKMζ, the isoform studied here, is an N-terminal truncated form of PKCζ which lacks the auto-inhibitory regulatory domain of the parent protein and contains only the catalytic domain (5). This makes it different from other forms of PKC by being constitutively active in the absence of the normal PKC signaling molecules (mainly calcium and diacylglycerol). Since PKMζ lacks most of the other isoforms' capacity for physiological regulation, it must function in an entirely different mode. The article by Shema et al. shows that PKMζ seems to enhance the learned response in taste-aversion conditioning. However, the authors also found that it enhanced the response to a weak aversive stimulus, whether it was given before training or after consolidation.
What do these results mean? One possibility is that PKMζ is somehow involved in heightening of the salience or the response component of the taste aversion response. This could explain how it can enhance multiple taste associations given at different times. Alternatively, PKMζ might be continuously required to maintain the strength of previously consolidated memories. This would imply that a constant input of energy (as ATP) is required to prevent forgetting. In the absence of kinase activity, there must be endogenous phosphatases that automatically and non-specifically erase consolidated memories. If this is so, much challenging work lies ahead as researchers discover how this system could enhance memories while still maintaining the learning-required stimulus specificity of distinct associations stored in long-term memory.
References:
Bank B, DeWeer A, Kuzirian AM, Rasmussen H, Alkon DL. Classical conditioning induces long-term translocation of protein kinase C in rabbit hippocampal CA1 cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1988-92. PubMed.
Olds JL, Anderson ML, McPhie DL, Staten LD, Alkon DL. Imaging of memory-specific changes in the distribution of protein kinase C in the hippocampus. Science. 1989 Aug 25;245(4920):866-9. PubMed.
Pasinelli P, Ramakers GM, Urban IJ, Hens JJ, Oestreicher AB, de Graan PN, Gispen WH. Long-term potentiation and synaptic protein phosphorylation. Behav Brain Res. 1995 Jan 23;66(1-2):53-9. PubMed.
Hongpaisan J, Sun MK, Alkon DL. PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer's disease transgenic mice. J Neurosci. 2011 Jan 12;31(2):630-43. PubMed.
Sacktor TC. How does PKMζ maintain long-term memory?. Nat Rev Neurosci. 2011 Jan;12(1):9-15. PubMed.
University College London
This study reports that learning in rats prompts their hippocampal astrocytes to release lactate, which is critical for retaining the acquired memories. Although memory formation has previously been shown to involve a myriad of generic metabolites, one exciting aspect of this paper is that it associates learning with the boost of astrocytic lactate release as an essential condition for downstream cascades in neurons (which are classically attributed to learning and memory). Furthermore, the enhanced lactate release appears important for long-term rather than short-term memory formation. Does this imply that astroglia receive a warning signal about long-term importance of a particular learning process before the memories are actually formed?
The results show that adding exogenous lactate could rescue memory impairment consequent to an interruption of astrocytic glycogenolysis activity. This suggests that memory loss associated with pathological deficiency in lactate supply might be improved by lactate, but whether such effects could be achieved for other brain dysfunctions resulting in memory impairment remains to be seen.
University of California, Los Angeles
In this important study, Shema et al. provide evidence that altering the activity of a single kinase in the brain, PKMζ, can have dramatic effects on the maintenance of long-term memory. Previous results from these same authors had reached similar conclusions (Pastalkova et al., 2006; Shema et al., 2007), but the earlier studies used drugs to inhibit PKMζ’s activity, leaving room for skepticism, because drugs can have non-specific effects. In this new study, the authors injected lentiviruses containing either the gene for PKMζ, or the gene for an inhibitory dominant-negative (DN) version of PKMζ, into the insular cortex (IC) of rats six days after the rats had been given conditioned taste aversion (CTA) training. (The IC is known to be the site of storage of the long-term memory for CTA [Yamamoto et al., 1980].) When tested seven days after the injections, rats whose IC contained neurons that overexpressed PKMζ due to viral infection exhibited significantly enhanced avoidance of the aversive taste. By contrast, in rats with IC neurons that overexpressed the DN form of PKMζ, the long-term memory for CTA was disrupted.
Importantly, the present study included several controls for non-specificity of the effects of their genetic manipulations. For example, the authors showed that overexpression of PKMζ in the IC did not affect the innate preference of untrained (naïve) rats for the taste of the substance (saccharin) used as the conditioned stimulus (CS) in CTA training; nor did IC overexpression of PKMζ change the volume of saccharin-laced liquid that rats consumed in the initial phase of CTA training, prior to the injection of lithium chloride (LiCl), the drug used to sicken the rats in the CTA protocol. Thus, the overexpression did not produce sensorimotor effects. The authors also showed that, despite producing enhancement of long-term memory, overexpression of PKMζ did not block normal extinction of CTA memory produced by repeated exposure to the CS. Another interesting finding in the present study is that the injection of the overexpression viral construct enhanced not only the aversive memory for a CS used in CTA training six days before the injection, but also the memory for a different CS used in an earlier round of CTA training eight days before the injection. Therefore, overexpression of PKMζ can enhance the CTA memory of multiple taste associations.
The study by Shema et al.—due to the specificity of the genetic manipulations that were used, together with the inclusion of extensive and appropriate controls—shrinks the room for skepticism regarding the importance of PKMζ in maintaining at least some forms of long-term memory to a vanishing point. Nonetheless, several key questions remain. Does altering PKMζ’s activity facilitate or disrupt general retrieval processes (“item-invariant” memory operations in the authors’ term), or does it strengthen or weaken specific stored memories (through “item-variant” memory operations)? The authors prefer the latter interpretation, but the data in their study do not decide the issue. Also, is PKMζ required for the maintenance of all long-term memories? Apparently not, because inhibition of PKMζ does not disrupt the retention of some forms of long-term memory (Shema et al., 2007; Serrano et al., 2008; Kwapis et al., 2009). What, then, are the other key memory-maintaining molecules? Finally, and most importantly, if PKMζ is indeed a key molecule for memory maintenance, as the present study indicates, exactly how does its activity mediate the persistence of specific memories? Some work has been done on this critical question. Sacktor and his colleagues have shown that PKMζ activity maintains GluR2 subunit-containing 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid (AMPA) receptors in the post-synaptic membrane of recently potentiated synapses (Migues et al., 2010). However, PKMζ may mediate memory persistence through other mechanisms. For example, long-term memory is known to involve structural changes in the nervous system (Bailey and Kandel, 1993). Might PKMζ’s activity play a role in the maintenance of these changes?
The results in the present study proffer the hope that we will one day be able to modify long-term memories. Such modification holds out hope for treatment of such memory-related disorders as Alzheimer’s disease, post-traumatic stress disorder, and drug addiction. The technology of memory modification is still a long way off, however. The development of this technology will require that we answer the questions outlined above, as well as develop the means to physically identify specific engrams in the human brain (see Han et al., 2007). Nonetheless, the study by Shema et al. represents a major step toward the eventual goal of manipulating long-term memory.
References:
Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397-426. PubMed.
Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007 Apr 20;316(5823):457-60. PubMed.
Kwapis JL, Jarome TJ, Lonergan ME, Helmstetter FJ. Protein kinase Mzeta maintains fear memory in the amygdala but not in the hippocampus. Behav Neurosci. 2009 Aug;123(4):844-50. PubMed.
Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010 May;13(5):630-4. PubMed.
Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006 Aug 25;313(5790):1141-4. PubMed.
Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, Yin JC, Sacktor TC, Fenton AA. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008 Dec 23;6(12):2698-706. PubMed.
Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007 Aug 17;317(5840):951-3. PubMed.
Yamamoto T, Matsuo R, Kawamura Y. Localization of cortical gustatory area in rats and its role in taste discrimination. J Neurophysiol. 1980 Sep;44(3):440-55. PubMed.
Make a Comment
To make a comment you must login or register.