Does TDP-43 Oligomerize and Coax Aβ to Do the Same?
Quick Links
TDP-43 self-assembles into oligomers that are able to recruit Aβ to form oligomers of its own, claims a paper in the September 12 Nature Communications. Using a new TDP-43 oligomer antibody, the authors report binding in postmortem brain samples from people who had frontotemporal dementia. Senior author Yun-Ru (Ruby) Chen of Academia Sinica in Taipei, Taiwan, suggests that these multimers may be toxic, as has been proposed for other oligomers implicated in neurodegeneration. The new antibody, or one like it, might help doctors diagnose or even one day treat TDP-43 proteinopathy, Chen speculated.
“Many people knew that these oligomers existed … this is the first comprehensive study,” noted Rakez Kayed of the University of Texas Medical Branch in Galveston (Johnson et al., 2009; Guo et al., 2011; Choksi et al., 2013).
The finding that TDP-43 causes Aβ to oligomerize is new, added Kayez, who was not involved in the current work. If confirmed, it would complement recent work by Kayez and collaborators showing that Aβ can template TDP-43 aggregation (Guerrero-Muñoz et al., 2014). Kayez hopes that Chen’s new findings will inspire researchers to examine TDP-43 oligomers, TDP-43 mutations, and their potential toxicity more closely in animal models.
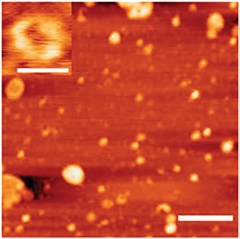
TDP-43 forms round oligomers, as seen by atomic force microscopy (scale bar=500 nanometers). In the upper left, a close-up of a ring-shaped oligomer (scale bar=50 nanometers). [Image courtesy of Fang et al., Nature Communications.]
TDP-43 Makes Round Oligomers
Chen and first author Yu-Sheng Fang have been working on TDP-43 biochemistry since researchers identified it as a major component in protein inclusions in FTD and ALS in 2006 (see Oct 2006 news story). They started by purifying recombinant human TDP-43 from Escherichia coli. While they expected their recombinant protein to produce a protein of the eponymous 43 kilodaltons, it immediately glommed together into structures more than 10 times that, according to size-exclusion chromatography. Because these aggregates were soluble, the researchers knew they were distinct from the filamentous, insoluble inclusion bodies that characterize FTD brain and ALS spinal cord.
Chen and Fang surmised they were looking at oligomers, and used the oligomer-specific antibody A11 to confirm their suspicions. A11 recognizes some conformation common to amyloid-forming oligomers regardless of the primary structure of the peptide, be it Aβ, α-synuclein, or other amyloidogenic protein (see Apr 2003 news story). Scientists still do not understand which epitope A11 recognizes, and some researchers get better results with it than others, noted David Teplow of the David Geffen School of Medicine at the University of California, Los Angeles, who was not involved in the work.
To investigate the oligomeric nature of TDP-43 more directly, Fang and colleagues used electron and atomic force microscopy to map them in three dimensions. The oligomers formed spherical and ring-shaped structures (see image above). They resembled previously characterized pathogenic Aβ and α-synuclein oligomers (Lashuel et al., 2002) but were larger, more than 50 nanometers across compared to two or three nanometers for typical Aβ oligomers. The TDP-43 structures also differed from Aβ in that they never adopted conformations that bound the dye thioflavin T, which binds to amyloid fibrils in beta sheet formation.
Next, Fang asked if oligomerization might alter TDP-43’s normal function as a DNA-binding protein. She compared the oligomers to a truncated TDP-43 that contained only the nucleic acid binding sites. While the shortened TDP-43 was able to attach itself to one of its target DNA sequences—transactivation response element from HIV—the oligomers bound the DNA more weakly. “That tells us the oligomer has a loss of the TDP-43 physiological function,” perhaps due to abnormal conformation or masking of the DNA-binding domain, Chen concluded.
The scientists also report evidence for a gain of toxic function. When Fang treated human neuroblastoma BE(2)-C cultures or primary mouse cortical neurons with the oligomers, about 20 percent of the cells died. The authors then injected oligomers into the hippocampus of live mice. Two weeks later, they examined the brains and observed a loss of cortical neurons where they injected the TDP-43.
Fang and Chen wondered if the TDP-43 oligomers they saw in vitro exist in the brains of people with FTD. To find out, they used the oligomers to create an antibody they called TDP-O. They claim that unlike A11, their antibody is specific for TDP-43 oligomers because it does not bind Aβ oligomers. They did not test TDP-O for reactivity against other amyloidogenic proteins, however, leading Teplow to suggest that it might still exhibit off-target binding. The authors bathed brain sections from FTD patients with TDP-O. Most TDP-43 antibodies detect fibrils, but not this one. Instead, it labeled oligomers that showed up as round structures under the electron microscope. When Fang and colleagues immunoprecipitated these structures with TDP-O, the structures also bound another TDP-43 antibody directed against the protein’s amino terminus, confirming they did indeed contain TDP-43.
TDP-43 Oligomers Attract Aβ
TDP-43 joins the ranks of many neurodegeneration-linked proteins that oligomerize, including Aβ, α-synuclein, and the prion protein PrP. These proteins cross-seed each other’s oligomerization (see Sep 2008 news story; Jul 2013 news story). Might TDP-43 do the same? Fang investigated by incubating TDP-43 oligomers with soluble Aβ. By itself, Aβ assembles into long fibrils. However, just a smidgen of TDP-43 inhibited that process, leading the authors to speculate that the Aβ got stuck in the oligomer stage. To check, they photo-crosslinked the Aβ and ran it on a protein gel. Without TDP-43, they saw Aβ monomers, dimers, trimers, and tetramers. After they added TDP-43, they observed Aβ pentamers and even larger species of uncertain makeup. In addition, when they looked at Aβ in the electron microscope, they saw it formed fibrils when alone, but only small orbs in the presence of TDP-43.
Chen cannot be sure what conformation these oligomers take, and whether they contain only Aβ or a combination of Aβ and TDP-43. Chen and Kayed agreed that this kind of cross-seeding may explain why TDP-43 inclusions are often present in AD brains (Amador-Ortiz et al., 2007; Uryu et al., 2008; Hu et al., 2008; Kadokura et al., 2009).
However, Teplow urged caution in interpreting these experiments. “I see no evidence of cross-seeding," he said, raising several caveats. The authors used Aβ40, not Aβ42, which is more relevant to Alzheimer’s disease, and they didn't use Aβ seeds as a positive control to ensure that they had the right conditions for seeding to occur. He also noted the transition from Aβ monomers to larger species did not rise proportionately with increasing TDP-43, suggesting the DNA binding protein may be superfluous. He agreed with the authors that TDP-43 seems to inhibit Aβ fibrillization, but was not convinced that it converted Aβ to oligomers.
Chen interprets her results to mean that TDP-43 oligomers are somehow involved in TDP-43 proteinopathies, and perhaps Alzheimer’s disease as well. She plans to investigate whether TDP-43-seeded Aβ species are toxic, and how the proposed cross-seeding occurs. For example, she would like to use the TDP-O antibody to look for TDP-43 oligomers in blood and cerebrospinal fluid from people with AD, ALS, and FTD and test if their levels correlate with phenotype, such as severity of symptoms.—Amber Dance
References
News Citations
- New Ubiquitinated Inclusion Body Protein Identified
- Amyloid Oligomer Antibody—One Size Fits All?
- Guilt by Association?—Aβ, α-Synuclein Make Mixed Oligomers
- An Extra Strain on the Brain—α-Synuclein Seeds Tau Aggregation
Paper Citations
- Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J, Gitler AD. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J Biol Chem. 2009 Jul 24;284(30):20329-39. Epub 2009 May 22 PubMed.
- Guo W, Chen Y, Zhou X, Kar A, Ray P, Chen X, Rao EJ, Yang M, Ye H, Zhu L, Liu J, Xu M, Yang Y, Wang C, Zhang D, Bigio EH, Mesulam M, Shen Y, Xu Q, Fushimi K, Wu JY. An ALS-associated mutation affecting TDP-43 enhances protein aggregation, fibril formation and neurotoxicity. Nat Struct Mol Biol. 2011 Jul;18(7):822-30. PubMed.
- Choksi DK, Roy B, Chatterjee S, Yusuff T, Bakhoum MF, Sengupta U, Ambegaokar S, Kayed R, Jackson GR. TDP-43 Phosphorylation by Casein Kinase I{epsilon} Promotes Oligomerization and Enhances Toxicity In Vivo. Hum Mol Genet. 2013 Nov 18; PubMed.
- Guerrero-Muñoz MJ, Castillo-Carranza DL, Krishnamurthy S, Paulucci-Holthauzen AA, Sengupta U, Lasagna-Reeves CA, Ahmad Y, Jackson GR, Kayed R. Amyloid-β oligomers as a template for secondary amyloidosis in Alzheimer's disease. Neurobiol Dis. 2014 Nov;71:14-23. Epub 2014 Aug 15 PubMed.
- Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT. Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002 Jul 18;418(6895):291. PubMed.
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007 May;61(5):435-45. PubMed.
- Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, Neumann M. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008 Jun;67(6):555-64. PubMed.
- Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, Parisi JE. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008 Aug;116(2):215-20. PubMed.
- Kadokura A, Yamazaki T, Lemere CA, Takatama M, Okamoto K. Regional distribution of TDP-43 inclusions in Alzheimer disease (AD) brains: their relation to AD common pathology. Neuropathology. 2009 Oct;29(5):566-73. PubMed.
Further Reading
Papers
- Kwong LK, Uryu K, Trojanowski JQ, Lee VM. TDP-43 proteinopathies: neurodegenerative protein misfolding diseases without amyloidosis. Neurosignals. 2008;16(1):41-51. PubMed.
- Bigio EH, Wu JY, Deng HX, Bit-Ivan EN, Mao Q, Ganti R, Peterson M, Siddique N, Geula C, Siddique T, Mesulam M. Inclusions in frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP) and amyotrophic lateral sclerosis (ALS), but not FTLD with FUS proteinopathy (FTLD-FUS), have properties of amyloid. Acta Neuropathol. 2013 Mar;125(3):463-5. PubMed.
- Miguel L, Frébourg T, Campion D, Lecourtois M. Both cytoplasmic and nuclear accumulations of the protein are neurotoxic in Drosophila models of TDP-43 proteinopathies. Neurobiol Dis. 2011 Feb;41(2):398-406. PubMed.
- Schwab C, Arai T, Hasegawa M, Yu S, McGeer PL. Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol. 2008 Dec;67(12):1159-65. PubMed.
News
- ALS-Linked TDP-43 Turns Amyloid in the Lab
- Toxic Synuclein Corrupts Native in Wild-Type Mice
- Synthetic Synuclein Corrupts Native Along Mouse Brain Networks
- Think Oligomers Are Bad? Think Again....
- Seeds of Destruction—Prion-like Transmission of Sporadic AD?
- Toxic TDP-43 Too Tough to Degrade, Plays Prion?
Primary Papers
- Fang YS, Tsai KJ, Chang YJ, Kao P, Woods R, Kuo PH, Wu CC, Liao JY, Chou SC, Lin V, Jin LW, Yuan HS, Cheng IH, Tu PH, Chen YR. Full-length TDP-43 forms toxic amyloid oligomers that are present in frontotemporal lobar dementia-TDP patients. Nat Commun. 2014 Sep 12;5:4824. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Drexel University
In this comprehensive and multifaceted study, Ruby Chen and her coworkers demonstrated that TDP-43, a protein associated with frontotemporal lobar dementia and amyotrophic lateral sclerosis, assembles into a heterogeneous population of quasispherical and annual oligomers, reminiscent of similarly shaped multimers known to play a critical role in other amyloidogenic disorders, such as Alzheimer's and Parkinson's diseases. The study shows that TDP-43 oligomers are toxic in vitro and in vivo, suggesting that a pathogenic mechanism akin to the mechanism postulated in other amyloid pathologies may be at play. TDP-43, however, performs an important function in RNA metabolism and its absence seems to be lethal at the embryonic stage. As TDP-43 oligomers relative to monomers exhibited reduced TDP-43 binding to DNA and RNA, this loss of function upon oligomerization may additionally contribute to the pathology.
I was not convinced about the interpretation of the PICUP/SDS-PAGE data, which led the authors to conclude that TDP-43 oligomers seed Aβ40 oligomerization. Their evidence is based on a TDP-43 concentration-dependent presence of a ~55 kDa species in the PAGE gel, as well as other species with a molecular mass above ~100 kDa. It is known that Aβ40 alone readily forms dimers through tetramers, while pentamer and hexamer bands are much less significant (Bitan et al., 2003). The interpretation of PICUP/SDS-PAGE data for a solution containing two proteins of very different lengths (40 and 414 residues comprising Aβ40 and TDP-43, respectively) is tricky, as discussed in a study that examined the mechanism of the C-terminal Aβ fragments as inhibitors of Aβ42 oligomer toxicity (Fradinger et al., 2008). During PICUP, tyrosine (and possibly other aromatic amino acids) are involved in covalent cross-linking. The species with a molecular mass of ~55 kDa could correspond to a covalently cross-linked complex of TDP-43 (with a molecular mass of 47 kDa) and Aβ40 dimer, for example. Given that TDP-43 is more than 10 times longer than Aβ40, this is a plausible scenario that would also explain why the presence of TDP-43 abolishes Aβ40 fibril formation, as Aβ40 peptides that form a stable complex with TDP-43 could not structurally convert into fibrils. This aspect of the study would benefit from further examination.
Detection and characterization of TDP-43 oligomers fits well into a general paradigm of a common structural motif across a broad class of amyloidogenic proteins which self-associate into heterogeneous quasispherical and/or annual oligomers, eventually forming fibers that can be detected by dyes such as Thioflavin T and Congo Red. From the point of view of statistical physics and thermodynamics, such a self-assembly pathway is not surprising, as we demonstrated recently by invoking a minimal model of self-assembly that captures universal assembly pathways from monomers through quasispherical oligomers into elongated multidomain fibril-like morphologies (Barz and Urbanc, 2014).
Considering that the population of TDP-43 oligomers Ruby Chen and her collaborators observed was heterogeneous and characterized by some degree of hydrophobic exposure, one would expect that these oligomers would further aggregate into larger species, although the corresponding structural conversion in TDP-43 might be significantly slower than in the case of the much shorter Aβ. Insights into this structural conversion process, if it indeed exists and can be unambiguously detected, might elucidate the missing link between the reported oligomers and the inclusion bodies, which are the pathologic hallmarks of frontotemporal lobar dementia.
References:
Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid beta -protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc Natl Acad Sci U S A. 2003 Jan 7;100(1):330-5. PubMed.
Fradinger EA, Monien BH, Urbanc B, Lomakin A, Tan M, Li H, Spring SM, Condron MM, Cruz L, Xie CW, Benedek GB, Bitan G. C-terminal peptides coassemble into Abeta42 oligomers and protect neurons against Abeta42-induced neurotoxicity. Proc Natl Acad Sci U S A. 2008 Sep 16;105(37):14175-80. PubMed.
Barz B, Urbanc B. Minimal model of self-assembly: emergence of diversity and complexity. J Phys Chem B. 2014 Apr 10;118(14):3761-70. Epub 2014 Mar 6 PubMed.
View all comments by Brigita UrbancMake a Comment
To make a comment you must login or register.