St. Moritz, Part 4. (It) Take(s) Five (To Make Aβ), New BACE Mice
Quick Links
Unlike prion researchers, their colleagues in Alzheimer’s disease have a rich trove of current and potential future drug targets into which they can sink their collective investigative teeth. It is, of course, the APP processing problem. Unlike PrP, APP is chopped up in a complex series of enzyme reactions, some of them right in the middle of a lipid bilayer, which involves three different proteases and generates the Aβ peptide as well as other fragments. The hope is that understanding this processing—which occurs in most cells of the body but over the course of decades leads to amyloid buildup only in the brain—will yield new treatment approaches beyond the ones that are currently in preclinical and clinical development.
In St. Moritz, Christian Haass of Ludwig-Maximilians University in Munich, Germany, took the audience on a tour, punctuated with plays on famous jazz compositions, of how far the field has come since the demonstration that presenilin is indeed the catalytic subunit of γ-secretase (Wolfe et al., 1999). Since then, it has become clear that the proteins nicastrin, aph-1, and PEN-2 join presenilin to form a γ-secretase complex. The cutting edge of research therefore has moved to ask, for example, just how the four arrange themselves into the unusual kind of enzyme that is an intramembraneous aspartyl protease. Another current question is whether this complex of four is complete or involves further, unknown players, especially in human neurons (Haass, 2004).
All four proteins in this quartet are equally important, and they keep tabs on each other in a process Haass dubbed “coordinated regulation.” Once this issue is fully resolved, it will probably be clear that all players interact with one another, Haass said. For now, his lab has established a sequence of protein-protein contacts and assembly that goes roughly like this: Immature nicastrin binds to aph1 and then folds itself properly. Next, presenilin binds nicastrin, and it does so by dipping its normally cytosolic C-terminus into the membrane and binding it to a transmembrane spot on nicastrin. Lastly, PEN-2 joins the trio. At this point presenilin is still inactive; it occurs in a pro-form until it gets cut through its long cytoplasmic loop and then comes together as an active heterodimer. Prior data from Takeshi Iwatsubo’s group suggested that PEN-2 might mediate this activating internal cut. Having recently tested this idea, Haass and his colleagues now suspect that the self-cleavage happens as an independent autoproteolytic event, and that PEN-2 then comes in to stabilize the emerging presenilin heterodimer (see Prokop et al., 2004). Where does all this happen? The γ-secretase quartet assembles itself in early areas of the membrane continuum that is the endoplasmic reticulum, Haass added. The complex does not move up to the plasma membrane to meet APP until it is fully put together.
The second question Haass addressed concerns whether the γ-secretase quartet is complete or needs further players. In parallel with other laboratories, his group tried to reconstitute γ-secretase activity outside of neurons, and finally pulled it off in yeast, which has no endogenous γ-secretase activity (Edbauer et al., 2003). “This is a simple model but it took five years to get it to work,” Haass said. Using this model, the researchers recovered Aβ38, 40, and 42. They also found that this yeast γ-secretase cleaves twice, once to release Aβ and then a second time to release the AICD fragment that is thought to translocate to the nucleus and regulate gene expression there. These two findings validate that the artificial yeast system recapitulates key characteristics of the neuronal γ-secretase, Haass said. All this does not imply that human γ-secretase acts as a foursome, Haass added, saying that many other proteins may well participate and modulate this reaction in vivo.
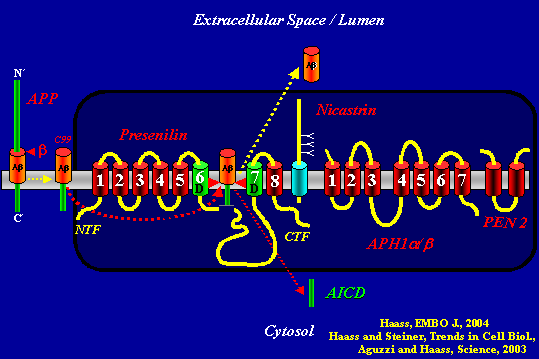
This slide summarizes the fate of APP when it is not cut by α-secretase. Dark box: APH1 binds Nicastrin, which binds the C-terminus of Presenilin. Next, PEN 2 binds to this trio and stabilizes the presenilin heterodimer that comes out of presenilin’s autoproteolysis of its long cytosolic loop. All this occurs in the ER. The complex then moves to the plasma membrane, where it meets APP. After b-secretase generates the C99 fragment from full-length APP, γ-secretase cuts C99 twice. First it releases Aβ into the extracellular space (or the lumen of an intracellular membrane compartment) and then AICD into the cytosol, from where it can signal to the nucleus.
Bart De Strooper of KU Leuven, Belgium, continued by saying that the yeast model was a technical feat, in part because it showed definitively the minimum requirements needed to get γ-secretase going. At the same time, human presenilin complexes likely assemble in a variety of forms. This real-life variability perhaps holds out hope for future, more specific γ-secretase drugs, especially if making a small dent in its activity were sufficient. “γ-secretase is a complicated activity and we are only beginning to understand it,” de Strooper said. He and Haass agreed that none of the current crop of inhibitors can separate APP from notch (see ARF related news story). Indeed, even partial PS knockouts de Strooper’s lab created to model γ-secretase inhibition more like a drug would do it and less like a complete deletion came down with autoimmune problems that one can expect if T cell maturation goes awry.
The γ-secretase variations—different complexes forming in different tissue, for example—may be necessary because the biological role of this enzyme is to help the degradation of a range of membrane proteins by cutting them right in their transmembrane sections. For example, mice harbor 3 Aph1 genes, and initial data indicate that conditional knockouts of Aph1a, b, and c generate different phenotypes. In a broader program, De Strooper’s lab is selectively deleting parts of the complex and monitoring the effect on APP and other substrates.
De Strooper’s postdoc Diana Dominguez presented initial data on their BACE1/2 double knockout mice. BACE is a favored drug target in part because single BACE knockout mice develop and age normally (see Luo et al., 2003). In practice, BACE may prove more recalcitrant, de Strooper said. For one, the protein is difficult to inhibit, though this is a technical hurdle the pharmaceutical industry may overcome in time. For another, new data suggests that inhibiting BACE might cause side effects, too. BACE 1 and 2 are the only members of this group of aspartyl proteases, and some data indicates that 2 might act to restrain 1. To get a better sense of how BACE1 and 2 interact, and of their role in vivo, Dominguez created mouse lines that lack BACE1, BACE2, or both.
The double knockout mice are fertile but, puzzlingly, half the litters die by three weeks of age. The scientists don’t yet know why, but early suspicion has fallen on germs in the mouse facility. Perhaps these double-knockouts are particularly sensitive to infection.
Finally, de Strooper outlined new research on a physiological function of APP. Studies in fruit flies indicate that it may help the brain recover from traumatic injury. Stay tuned for more on this story later.—Gabrielle Strobel
References
News Citations
Paper Citations
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999 Apr 8;398(6727):513-7. PubMed.
- Haass C. Take five--BACE and the gamma-secretase quartet conduct Alzheimer's amyloid beta-peptide generation. EMBO J. 2004 Feb 11;23(3):483-8. PubMed.
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003 Mar 27;422(6930):438-41. PubMed.
- Prokop S, Shirotani K, Edbauer D, Haass C, Steiner H. Requirement of PEN-2 for stabilization of the presenilin N-/C-terminal fragment heterodimer within the gamma-secretase complex. J Biol Chem. 2004 May 28;279(22):23255-61. PubMed.
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003 May;5(5):486-8. PubMed.
- Luo Y, Bolon B, Damore MA, Fitzpatrick D, Liu H, Zhang J, Yan Q, Vassar R, Citron M. BACE1 (beta-secretase) knockout mice do not acquire compensatory gene expression changes or develop neural lesions over time. Neurobiol Dis. 2003 Oct;14(1):81-8. PubMed.
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.