Does ALS Gene Police RNA, Keep Strands From Entangling?
Quick Links
Like a knot in your shoelace, entwined mRNAs can interfere with the nucleic acid’s normal actions. In amyotrophic lateral sclerosis, could it be that the cell overreacts to excess RNA snarls by responding with a toxic anti-virus response? Tassa Saldi of the University of Colorado in Boulder put forth this theory at the Keystone Symposium “New Frontiers in Neurodegenerative Disease Research,” held 4-7 February 2013 in Santa Fe, New Mexico. She studied the Caenorhabditis elegans orthologue of the ALS gene TDP-43, called TDP-1. Unlike TDP-43-negative mammals, C. elegans lacking TDP-1 survive, allowing her to examine the downstream effects on mRNAs.
“Their study was a great example of the importance of simple model organisms in learning about the normal function of disease proteins,” commented Aimee Kao of the University of California, San Francisco, in an e-mail to Alzforum. “It will hopefully stimulate some new thinking about the function of TDP-43 and how mutations cause disease.”
TDP-43 regulates expression and splicing of thousands of RNAs. To understand TDP-1’s role in the nematodes, Saldi, who works in the laboratory of Christopher Link, sequenced the transcriptome of the deletion strain. She discovered that 1,200 transcripts were over- or underexpressed compared to normal worms, and 350 genes were differentially spliced. No major gene categories emerged from the gene set, however, leaving few hints as to TDP-1’s primary effects.
Going through the list of genes one by one, Saldi did discover a common theme. It was gene overlap, a phenomenon where a given nucleotide sequence in one gene is also expressed as part of another gene (Sanna et al., 2008). Many genes differentially regulated in TDP-1’s absence overlapped with other genes, and the common sequences ran in opposite directions. This could occur if genes on opposite DNA strands share antiparallel coding sequences. It could also happen when one gene’s very long intron contains a second gene. This is not the first time long introns have been linked to TDP pathology; extended introns in the mouse genome are among the top targets of TDP-43 activity (see ARF related news story on Polymenidou et al., 2011, and Tollervey et al., 2011).
In general, about 8 percent of the worm genome overlaps, Saldi said. In the 1,550 genes that depend on TDP-1 for proper regulation, 35 to 45 percent were overlappers. The dataset also contained an unusually high number of introns of three kilobases or longer.
How does the TDP-1 knockout affect overlapping genes? If both sides of the DNA are transcribed at the same time, then their mRNAs are at risk of annealing to form a double-stranded structure. In fact, loss of TDP-1 resulted in noticeable dsRNA buildup in the worms, Saldi found. She stained the animals or individual tissues with an antibody to dsRNA and observed large nuclear inclusions. She could clear those aggregates by adding double-strand-specific RNase, but not RNase for single-stranded RNA, confirming their double-stranded structure.
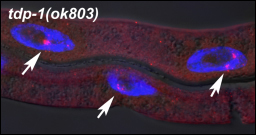
Double-stranded RNAs, labeled with an antibody (red), accumulate in the nuclei of C. elegans lacking TDP-1. Image courtesy of Tassa Saldi, University of Colorado, Boulder
Saldi hypothesized that TDP-1 might work in RNA editing, which has evolved to disrupt these dsRNAs. Adenosine deaminases swap adenines for inosines, which pair awkwardly with the guanine on the opposite strand, forcing the dsRNA to unwind. This repair typically happens in untranslated regions or introns, and so does not interfere with the protein code, Saldi said.
When Saldi examined the editing system in her animals, she observed that the adenine-to-inosine transition still occurred in the transcriptome of TDP-1-negative worms. In fact, the deletion strain had more inosines than normal. Therefore, the editing process is working properly, Saldi said. She suspects that TDP-1 normally acts upstream, destabilizing dsRNAs so they unwind without editing. Worms lacking TDP-1, then, would require extra editing. Another possibility, she added, is that TDP-1 participates in dsRNA degradation.
Worms, of course, are not people, but Saldi found hints that TDP-43 in human cells performs a similar function. When she knocked down TDP-43 in HeLa cervical cancer cultures, dsRNA inclusions formed in the nucleus. Similarly, dsRNA inclusions formed in TDP-43-deficient M17 neurons, but this time the inclusions were cytoplasmic.
How might dsRNA aggregates contribute to neurodegeneration? Double-stranded RNA in the cytoplasm should raise a red flag, said Saldi, because it could be evidence of infection with a dsRNA virus. A natural response would be the interferon immune pathway that fights infection, but this can also be toxic. Could that be how TDP loss damages neurons? Supporting this theory, Saldi noted that many of TDP-43’s mRNA targets are involved in the interferon response (Polymenidou et al., 2011).—Amber Dance.
References
News Citations
Paper Citations
- Sanna CR, Li WH, Zhang L. Overlapping genes in the human and mouse genomes. BMC Genomics. 2008;9:169. PubMed.
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling SC, Sun E, Wancewicz E, Mazur C, Kordasiewicz H, Sedaghat Y, Donohue JP, Shiue L, Bennett CF, Yeo GW, Cleveland DW. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011 Apr;14(4):459-68. PubMed.
- Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M, König J, Hortobágyi T, Nishimura AL, Zupunski V, Patani R, Chandran S, Rot G, Zupan B, Shaw CE, Ule J. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci. 2011 Apr;14(4):452-8. PubMed.
Further Reading
Papers
- Arnold ES, Ling SC, Huelga SC, Lagier-Tourenne C, Polymenidou M, Ditsworth D, Kordasiewicz HB, McAlonis-Downes M, Platoshyn O, Parone PA, Da Cruz S, Clutario KM, Swing D, Tessarollo L, Marsala M, Shaw CE, Yeo GW, Cleveland DW. ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):E736-45. PubMed.
- Belzil VV, Gendron TF, Petrucelli L. RNA-mediated toxicity in neurodegenerative disease. Mol Cell Neurosci. 2012 Dec 29; PubMed.
- Narayanan RK, Mangelsdorf M, Panwar A, Butler TJ, Noakes PG, Wallace RH. Identification of RNA bound to the TDP-43 ribonucleoprotein complex in the adult mouse brain. Amyotroph Lateral Scler Frontotemporal Degener. 2012 Oct 24; PubMed.
- Vaccaro A, Tauffenberger A, Ash PE, Carlomagno Y, Petrucelli L, Parker JA. TDP-1/TDP-43 regulates stress signaling and age-dependent proteotoxicity in Caenorhabditis elegans. PLoS Genet. 2012 Jul;8(7):e1002806. PubMed.
- Zhang T, Hwang HY, Hao H, Talbot C, Wang J. Caenorhabditis elegans RNA-processing Protein TDP-1 Regulates Protein Homeostasis and Life Span. J Biol Chem. 2012 Mar 9;287(11):8371-82. PubMed.
- Ash PE, Zhang YJ, Roberts CM, Saldi T, Hutter H, Buratti E, Petrucelli L, Link CD. Neurotoxic effects of TDP-43 overexpression in C. elegans. Hum Mol Genet. 2010 Aug 15;19(16):3206-18. PubMed.
News
- TDP-43 Controls Blood Vessels in Fish, Is Phosphorylated in Worms
- ALS-Linked Ataxin Repeats Stick It to TDP-43 in Stressed Cells
- San Diego: TDP-43 Targets Loom Large—But Where’s the Bull’s Eye?
- RNA Processors Turn Up in Another Motor Neuron Disease
- Good Prion, Bad Prion: TDP-43 Domain Plays Both Sides
- London, Ontario: TDP-43 Across the Animal Kingdom at ALS Meeting
- CLIPs of TDP-43 Provide a Glimpse Into Pathology
- Exploring Genome Fragmentation in Neurodegeneration
- Up-and-Coming ALS Mice Leave Scientists ConFUSed
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.