Meningeal Cuffs Around Veins Form Exit and Entry Ramps to the Brain
Quick Links
Cerebrospinal fluid washes waste from the brain, eventually draining into the lymph nodes and bloodstream—but exactly how this flow bypasses the brain’s barriers to reach the periphery has remained somewhat mysterious. In the February 7 Nature, researchers led by Jonathan Kipnis, at Washington University in St. Louis, report that CSF exits along the outside of veins that connect the parenchyma to the dura mater lining the skull. These so-called bridging vessels (image below) require small openings in the arachnoid mater layer, which otherwise forms an impermeable sheath around the brain.
- The arachnoid mater forms cuffs around veins, creating gaps in the barrier.
- Cerebrospinal fluid exits the brain through these gaps, reaching dura mater.
- In disease, immune cells from the dura enter the brain through the same portals.
To visualize these gaps, the authors generated transgenic mice that have all of their arachnoid barrier cells labeled with a green fluorescent protein. Lo and behold, these cells formed “cuffs” around bridging veins. With live imaging, the authors traced molecules injected into the CSF as they flowed through these arachnoid cuff exit (ACE) points to reach the dura. What's more, traffic went the other way, too. Under neuroinflammatory conditions, immune cells traveled from the dura along these highways into the brain.
“The central nervous system space is physically connected to the dura mater through these gaps in the arachnoid barrier,” Kipnis and first author Leon Smyth wrote to Alzforum.

Brain Highways. In mouse brain, an ovalbumin tracer (gold) injected into the cerebrospinal fluid exits the brain (bottom) along bridging veins that lead to the dura mater (top). It collects in the superior sagittal sinus (SSS). [Courtesy of Smyth et al., Nature.]
Other researchers were enthusiastic. “This elegant study significantly contributes to the resolution of a long-debated question regarding the communication between the subarachnoid spaces and the dura,” wrote Michal Schwartz, Javier Peralta Ramos, and Giulia Castellani at the Weizmann Institute of Science in Rehovot, Israel. Kipnis previously studied with Schwartz. Per Kristian Eide at the University of Oslo, Norway, called the findings a potential breakthrough. “[The paper] introduces fresh insights into critical gaps in our existing knowledge,” he wrote to Alzforum.
Many saw broad applications. “The identification of these ACE points has potential implications not just for better understanding of CSF physiology, but also for monitoring disease progression, immune surveillance of the CNS, and immune reactivity,” wrote Berislav Zlokovic and Kassandra Kisler at the University of Southern California in Los Angeles (comments below).
At the same time, researchers noted that it remains to be determined how much of the brain’s CSF outflow goes through ACE points versus other hypothesized routes.
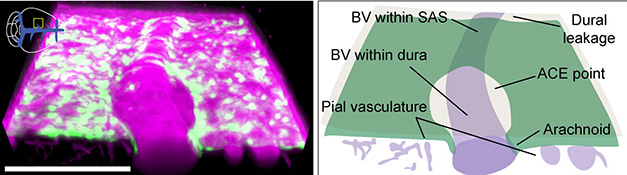
Gaps in the Barrier. Bridging veins (BV) poke through the arachnoid mater (green), allowing dextran tracer (pink) to flow from the subarachnoid space (SAS) to the dura. [Courtesy of Smyth et al., Nature.]
New Route for Brain Access
Tracer studies going back decades have found that CSF drains from inside the brain to cervical lymph nodes, under the ears (McComb, 1983; Eide et al., 2018). More recently, researchers discovered lymphatic vessels in the dura mater of both mice and people, implying these might be the initial collection point for CSF before it proceeds into nearby nodes (Oct 2017 news; Nov 2017 news; Jacob et al., 2022). How CSF reaches the dural lymphatic system was less clear. Theories included that it exits the brain alongside cranial nerves, or that it reaches the periphery via arachnoid granulations, small outpouchings of the arachnoid mater that contact the dura (Upton and Weller, 1985; Gailloud et al., 2001; Spera et al., 2023). The issue remains unsettled.
Direct Connection to Dura? Tracer (purple) injected into mouse cerebrospinal fluid appears first in the dura (left), then the cervical lymph nodes (middle), and finally the bloodstream (right). [Courtesy of Smyth et al., Nature.]
To take a closer look at CSF dynamics, Smyth injected the tracer Evan’s blue into the CSF of wild-type mice, and watched where it went via live imaging. The tracer appeared first in the dura, later in cervical lymph nodes, and last in blood (see video above). Smyth and colleagues confirmed these findings by taking lysates from each tissue and measuring tracer concentration at various time points. Its rapid emergence into the dura suggested to them the existence of a direct route between brain and meninges.

Arachnoid Cuff. Arachnoid barrier cells (pink) form cuffs on bridging veins (green) that lead to the dura. Endothelial junctions are blue. [Courtesy of Smyth et al., Nature.]
How did Evan’s blue reach the dura? Not by active transport through the arachnoid mater, the scientists think, because a single-nuclei RNA-Seq analysis of barrier cells found few transporters in these cells. Looking closer at the arachnoid, immunostaining of barrier cells revealed them bunched up in cuffs, or ACE points, around bridging veins (see image at right).
To visualize what happens at these points in live mice, the authors generated transgenics with fluorescent arachnoid barrier cells, using the marker Dpp4, which has been shown to be specific for these cells (Sep 2023 news). In live imaging, dextran injected into the CSF moved along bridging veins. Once the tracer passed the ACE point, it spread out into the dura (see video and image below). There, dextran accumulated in “hot spots” near venous sinuses, the channels that collect oxygen-depleted blood. Lymphatic vessels run alongside these sinuses.
Beyond the Gate. Dextran (purple) injected into mouse CSF flows along bridging veins (black) past the arachnoid cuff (green) before spreading into the dura mater (skull is turquoise). [Courtesy of Smyth et al., Nature.]
If molecules can exit the brain through ACE points, do they also enter there? To test this, the authors applied a type of biotin to the surface of the skull in transgenic Dpp4 mice. It diffused through to the dura, where it accumulated around bridging veins. Ten minutes later, it had reached the subarachnoid space.
ACE points permit access to the brain, the authors concluded. This may explain how immune cells in the meninges can communicate with the brain via cytokines (Oct 2019 news). Nonetheless, gatekeepers may still police these entrances. The authors noted that macrophages gather at ACE points and could devour unwanted visitors.

Time Course. Via live imaging of mouse brain (left), researchers watch dextran (purple) injected into mouse CSF flow up a bridging vein past an ACE point (green) and into the dura. Skull is turquoise. [Courtesy of Smyth et al., Nature.]
Most commenters found the data for ACE points convincing, saying the study affords the best look yet at the structure of the arachnoid mater around bridging veins. “This important blood-brain border has been overlooked, mostly because of a lack of tools to dissect its anatomy and cellular and functional components,” Tal Iram at the Weizmann Institute wrote to Alzforum. Sandro Da Mesquita at the Mayo Clinic in Jacksonville, Florida, praised the state-of-the-art imaging techniques. He previously worked with Kipnis. Julie Siegenthaler at the University of Colorado Anschutz Medical Campus, Aurora, called the study the most extensive analysis to date of these regions. “This … provides new tools and an important framework for numerous future studies on how these [ACE points] are altered in aging and disease,” she wrote.
Christer Betsholtz at Uppsala University, Sweden, cautioned that much of the evidence for brain-dura communication remains indirect, and alternate explanations are possible. For example, after tracer injection into the CSF, a small amount may exit the brain along cranial nerves, enter the bloodstream, and reach dura that way. Betsholtz noted that tracer in these experiments accumulated in macrophages that cluster around dural sinuses. These cells may concentrate the signal enough for it to be detectable in the dura before enough tracer has built up in the bloodstream to be visible there. In keeping with this, tracer hot spots in the dura co-localized better with macrophage abundance than with ACE points, he said. “Molecular and cellular passage along bridging veins and ACEs remains hypothetical. More work needs to be done to prove or disprove it,” Betsholtz wrote to Alzforum.
Do ACE Points Control Access for Immune Cells, Molecules?
Several recent papers have reported that immune cells enter the brain in various disease conditions (Jan 2020 news; Jun 2021 news). However, in the healthy brain, the dura harbors a distinct population of immune cells, containing more monocytes, neutrophils, B cells, and T cells than does the subarachnoid space. What keeps them from passing through ACE points into the brain?
Examining their snRNA-Seq dataset, the authors found that dural border cells that reside directly above the arachnoid mater express numerous secreted chemorepellent factors. In the healthy brain, these might well drive immune cells away from the arachnoid layer, the authors speculated. Supporting this idea, they found that two of these repellents, SEMA3A and SEMA3D, kept monocytes at bay in vitro. Knocking down SEMA3A in mouse meninges increased the number of monocytes found in the subarachnoid space.
Does anything change in the diseased brain? To investigate this, the authors used mice with experimental autoimmune encephalomyelitis (EAE), a neuroinflammatory model of multiple sclerosis. Compared with healthy mice, EAE mice expressed less SEMA3A in the meninges, and had more neutrophils and T cells in their subarachnoid space. In addition, live imaging showed myeloid cells moving along bridging veins into the subarachnoid space (see video below).
Immune Migration. In the EAE mouse brain, myeloid cells (green) move down along a bridging vein (purple) toward the subarachnoid space; yellow lines show their trajectories. [Courtesy of Smyth et al., Nature.]
Hints From Human Data
There is some evidence ACE points could be present in human brain, as well. The authors analyzed MRI data from 10 healthy volunteers participating in a multiple sclerosis study. Contrast agent injected into the bloodstream accumulated around bridging veins in the dura, suggesting the existence of the same brain-dura pathway as in mice (see image below). A previous MRI study showed that tracer injected into CSF entered the dura near the sites of bridging veins, implying that CSF can flow out along this pathway (Ringstad and Eide, 2020). More recently, a pilot study that used ultrasound to open the blood-brain barrier in Alzheimer’s disease patients found tracer accumulating around dural vessels, as well (Mehta et al., 2023).
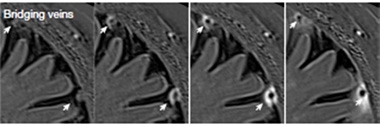
In Humans, Too. MRI of human brain shows contrast agent (white), injected into the bloodstream at baseline (left), accumulating around bridging veins (arrows) in the dura 15 (middle left), 45 (middle right), and 240 (right) minutes later. [Courtesy of Smyth et al., Nature.]
“The concept of ACE points is concordant with recent in vivo human data … This conception of CNS fluid flow updates knowledge on brain-body connections and physiology that has historically been perplexing,” Rupal Mehta at Rush Medical College, Chicago, wrote to Alzforum (comment below).
Commentators wondered about implications for treatment. “Can ACE points be used therapeutically to deliver drugs to the CSF and ultimately the CNS, as an alternative to intrathecal delivery?” Siegenthaler asked. Iram thinks this is worth exploring. “[This] entry point … should catch the attention of pharmaceutical companies that have been focusing mostly on the blood-brain barrier as an entryway for therapeutics,” she wrote.—Madolyn Bowman Rogers
References
News Citations
- Lymphatic Vessels Found in Human Brain
- In Mice, CSF Caught Draining Via Lymphatic Vessels, Not Veins
- Not So Fast—The Brain Has Three Meningeal Membranes After All
- Do Immune Cells in the Meninges Help with … Memory?
- Attack of the Clones? Memory CD8+ T Cells Stalk the AD, PD Brain
- Private Stock—Brain Taps Skull Bone Marrow for Immune Cells
Paper Citations
- McComb JG. Recent research into the nature of cerebrospinal fluid formation and absorption. J Neurosurg. 1983 Sep;59(3):369-83. PubMed.
- Eide PK, Vatnehol SA, Emblem KE, Ringstad G. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci Rep. 2018 May 8;8(1):7194. PubMed.
- Jacob L, de Brito Neto J, Lenck S, Corcy C, Benbelkacem F, Geraldo LH, Xu Y, Thomas JM, El Kamouh MR, Spajer M, Potier MC, Haik S, Kalamarides M, Stankoff B, Lehericy S, Eichmann A, Thomas JL. Conserved meningeal lymphatic drainage circuits in mice and humans. J Exp Med. 2022 Aug 1;219(8) Epub 2022 Jul 1 PubMed.
- Upton ML, Weller RO. The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations. J Neurosurg. 1985 Dec;63(6):867-75. PubMed.
- Gailloud P, Muster M, Khaw N, Martin JB, Murphy KJ, Fasel JH, Rüfenacht DA. Anatomic relationship between arachnoid granulations in the transverse sinus and the termination of the vein of Labbé: an angiographic study. Neuroradiology. 2001 Feb;43(2):139-43. PubMed.
- Spera I, Cousin N, Ries M, Kedracka A, Castillo A, Aleandri S, Vladymyrov M, Mapunda JA, Engelhardt B, Luciani P, Detmar M, Proulx ST. Open pathways for cerebrospinal fluid outflow at the cribriform plate along the olfactory nerves. EBioMedicine. 2023 May;91:104558. Epub 2023 Apr 10 PubMed.
- Ringstad G, Eide PK. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat Commun. 2020 Jan 17;11(1):354. PubMed.
- Mehta RI, Carpenter JS, Mehta RI, Haut MW, Wang P, Ranjan M, Najib U, D'Haese PF, Rezai AR. Ultrasound-mediated blood-brain barrier opening uncovers an intracerebral perivenous fluid network in persons with Alzheimer's disease. Fluids Barriers CNS. 2023 Jun 16;20(1):46. PubMed.
External Citations
Further Reading
Primary Papers
- Smyth LC, Xu D, Okar SV, Dykstra T, Rustenhoven J, Papadopoulos Z, Bhasiin K, Kim MW, Drieu A, Mamuladze T, Blackburn S, Gu X, Gaitán MI, Nair G, Storck SE, Du S, White MA, Bayguinov P, Smirnov I, Dikranian K, Reich DS, Kipnis J. Identification of direct connections between the dura and the brain. Nature. 2024 Mar;627(8002):165-173. Epub 2024 Feb 7 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Weizmann Institute of Science
Weizmann Institute of Science
Weizmann Institute of Science
Over the last 20 years, it became evident that adaptive and innate immune cells play a key role in lifelong brain plasticity and protection in health, with far-reaching implications for brain aging and diseases. This transformative understanding raised questions regarding brain anatomy, specifically, how these cells attain access to the brain in health, and how interactions with immune cell populations are modified under disease conditions. This elegant study from the Kipnis lab significantly contributes to the resolution of a long-debated question regarding the communication between the subarachnoid spaces and the dura.
Kipnis' group reports the identification of previously unknown gateways between the dura and the brain, coined arachnoid cuff exit (ACE) points. These enable the direct efflux of CSF from the subarachnoid space to the dura and orchestrate immune cell trafficking in the reverse direction. The authors further suggest that similar structures are present in humans. The evidence for such a route and its mechanisms of regulation represent a noteworthy advance in the understanding of communication of the brain with the immune system.
This is an exciting discovery for the field of brain immunity, because it helps address one of the biggest mysteries regarding the brain-immune interplay, which has remained enigmatic since we first showed that the brain is dependent on immune cells for its plasticity. Kipnis was a student in my lab at that time, and he subsequently demonstrated that immune cells residing in the meninges can remotely affect the brain. Nevertheless, it was not understood how these cells were regulated.
Overall, the discovery of ACE points provides a mechanism for the brain's protection from an unwanted or excessive immune response, and could also provide additional potential therapeutic targets under various brain pathologies.
Oslo University Hospital / University of Oslo
This paper is of great importance. It potentially represents a breakthrough in our comprehension of how substances in the CSF of the subarachnoid space communicate with the dura mater, which houses lymphatic vessels. It introduces fresh insights into critical gaps in our existing knowledge, addressing questions such as the anatomic efflux routes of CSF from the subarachnoid space, the entry of immune cells and products from the peripheral circulation to the CSF and brain, and the permeability of the arachnoid membrane.
The paper is also relevant to debated aspects of the glymphatic concept, including the connection between the proposed pial perivenous efflux site and the dural lymphatic structures. These inquiries are crucial for comprehending CSF physiology.
The work by Smyth et al. uncovered discontinuities in the arachnoid barrier, where bridging veins from the brain traverse the arachnoid membrane, entering the dura mater. These discontinuities create perivenous openings denoted as arachnoid cuff exit (ACE) points, facilitating the passage of substances between the subarachnoid space and the dura. Consequently, locations where bridging veins enter the dura serve as focal points for CSF efflux.
The potential implications of ACE sites are broad, enabling the exchange of cells between the dura and CSF in the subarachnoid space. Importantly, ACE points were also identified as entry points for substances from veins to the CSF, shedding light on the discussion of how molecules from the systemic circulation, such as intravenous contrast agents, enter the CSF.
This recent paper raises several questions: What is the relative importance of ACE points for CSF efflux? Is CSF efflux at the ACE sites actively controlled, or do these sites represent passive efflux pathways where CSF bulk flow is primarily regulated by hydrostatic and osmotic pressure gradients?
University of Southern California
University of Southern California, Keck School of Medicine
Smyth et al. present several new observations about the nature and function of the meningeal layers surrounding the brain. Using transcriptomic data, they generated new mouse models to visualize and study anatomical structures that function as routes across the arachnoid barrier. Although recent work has demonstrated that cells in the leptomeningeal layers surrounding the brain survey and detect molecular cues in the cerebrospinal fluid (CSF), how the fluid, molecules, and immune cells move between layers of the meninges remain open questions. Smyth et al. describe arachnoid cell “cuffs” around venous vessels, which, they show, control transport of solutes and immune cells between the subarachnoid space and the dura, and regulate the drainage of cerebrospinal fluid from brain. At the same time, these cuff points limit transport of molecules from the dura to the subarachnoid space.
The identification of these arachnoid cuff exit (ACE) points has potential implications not just for better understanding of the CSF system physiology, but also for monitoring disease progression, immune surveillance of the CNS, and immune reactivity. Indeed, the authors observed increased immune cell transits via these cuff points in an experimental autoimmune encephalomyelitis (EAE) model. In their model, the authors did not focus on whether the cuff points themselves underwent any changes in structure, function, or composition with EAE. However, it was previously reported that inflammatory conditions can alter the structure of the meningeal layers (Mapunda et al., 2023; Pietilä et al., 2023), making this an important question for future investigation.
How large a role the ACE route plays in the human CNS and in other diseases requires more research. That said, these cuff points may also open new avenues to therapeutic strategies if drug delivery into the CNS can be realized via these transit points, and if this is more effective than crossing the blood-brain barrier. Future studies should also investigate how much of a role these cuff points play in CNS fluid and cell movement and in CNS immune surveillance compared to other possible routes for exchange of solutes and cells in the brain.
Weizmann Institute of Science
The extent of novel anatomical observations at the leptomeningeal-brain interface coming out of this lab is astounding. This important blood-brain border has been overlooked, mostly because of lack of tools to dissect its anatomy and cellular and functional components. In this paper, the Kipnis lab combined single-cell transcriptomics with very carefully timed in-vivo imaging to identify subsets of leptomeningeal barrier cells and the way CSF flows between them.
I think that as with the discovery of CSF efflux through the brain lymphatics, this newly discovered exit route offers novel ways to manipulate CSF flow and debris clearance in aging and neurodegenerative diseases.
Furthermore, that this is also an entry point for molecules and immune cells from the blood to the brain (through the CSF) should catch the attention of pharmaceutical companies that have been focusing mostly on the blood-brain barrier as an entry way for therapeutics.
Mayo Clinic
This major scientific study coming from the Kipnis lab shines light onto the architecture of the mammalian meninges, and its crucial roles in mediating the molecular and cellular communication between the brain, its border tissues, and the periphery.
Despite the growing amount of data showing that cerebrospinal fluid molecular content can reach the outermost meningeal dura, and ultimately be drained by the dural lymphatics into the cervical lymph nodes, we had a very limited idea, and even less data, on how and where this communication takes place. The experimental evidence supporting the existence of anatomical regions in the mammalian meningeal arachnoid layer that mediate this communication between brain fluids, the meningeal dura and later the peripheral lymphatic and immune systems is finally here.
Using very elegant experimental mouse models and techniques, combined with human biospecimen analyses and state-of-the-art imaging modalities, this study was able to provide strong evidence for the existence of a rapid exchange, by a somewhat direct route, of molecular and cellular content between the subarachnoid space and the dura, via what the authors call arachnoid cuff exit points. Basically, the authors show that these cuffs in the arachnoid layer are due to penetrating bridging veins that cross it on their way to the dural venous sinuses. At these anatomical points the arachnoid layer barrier cells cannot form a tight barrier, and the arachnoid becomes permissive, allowing the passage of both molecules and immune cells into the dura.
The authors point out, and I totally agree, that this discovery opens new avenues of research in the field of neuroimmunology. For example, it will be important to understand the contribution of the arachnoid cuff exit points to CSF outflow into the dura and lymphatic system, when compared to the arachnoid villi-to-venous outflow pathways that are found in human arachnoid granulations.
It will also be interesting to look at how these arachnoid cuffs change, cellularly and structurally, in aging and in different aging-related diseases, where CSF composition and turnover are known to be altered, meningeal lymphatic drainage is poor, and neuroinflammation is a major component. The authors provide evidence suggesting that the arachnoid cuffs serve as “a way into” the subarachnoid and cerebrospinal fluid for dura-derived molecules and immune cells in models of central nervous system autoimmunity. So one would assume that, at some point during disease initiation, there is a shift in the flow directionality of molecules and immune cells via the arachnoid cuffs. It will be interesting to explore the mechanisms behind it, and whether this directional shift in flow through these cuffs around the bridging veins is also observed in aging-related chronic pathological conditions.
University of Colorado, Anschutz Medical Campus
This new work shows extensive anatomical and functional evidence that spots where bridging veins cross from the leptomeninges to the dura are entry-exit points for small and large molecular weight tracers. This is in contrast to the arachnoid barrier layer, shown in the past, and confirmed in this study, to occlude paracellular transport via presence of tight junctions.
The authors perform extensive fixed tissue and intravital imaging analysis using existing (Cdh5-CreERT and Prox1-GFP) and newly generated Cre lines (Dpp4-CreER and Slc47a1-CreER) to visualize arachnoid barrier (AB) and other leptomeningeal and dural fibroblast populations. This permits an extensive characterization of the cellular arrangements around these exit points, which alternatively appear as gaps in the AB layer or “cuffs” of AB cells as the vein exits the leptomeninges into the dura.
This is the most extensive analysis to date of these regions and provides new tools and an important framework for future studies on how these arachnoid cuff exits (ACE) are altered in aging and disease. Importantly, future studies targeting ACE points to disrupt or block flow of molecules will determine their functional relevance in brain health, permitting these and other questions to be answered:
The authors use their methods to study ACE points as potential entry sites for immune cells into the subarachnoid space in healthy mice and in an animal model of multiple sclerosis (EAE). Their data in Figure 5j,n show convincing evidence with intravital imaging at peak EAE that monocytes are moving from the supra-arachnoid (marked by Prox1) indicative of the dura, to SAS, below the Prox1-GFP layer, using the ACE-bridging vein as an entry point.
However, regarding the extravasation of monocytes from leptomeningeal vasculature that occurs in neuroinflammation, it is difficult to discern if this represents an entry point that substantially contributes to disease pathology. The authors do provide functional data to address this point by zeroing in on integrin-alpha6 (expressed by immune cells and endothelial cells) as a potential receptor-extracellular matrix interaction that is permissive to ACE point crossing, similar to what was described by Dorothy Sipkins and colleagues for cancer cell access to the SAS (Yao et al., 2018).
The authors show, using systemic administration of anti-itga6, that clinical disease score is reduced and neutrophil density at ACE points is lower, but the total immune cell entry into the spinal cord shows some mixed results. While clearly of interest and an important avenue of research, more studies are needed to interrogate how immune cell entry via ACE points contributes to disease progression versus other routes of entry, such as across the vasculature. Here, too, functional studies to block ACE points will be valuable.
While I recognize this is beyond what the authors discuss, and not the focus of this study, I'd like to draw attention to the electron microscopy studies following HRP injection into the cisternal magna to target the SAS. They are particularly interesting, and relevant to recent discussions of meningeal anatomy and function, including reported barrier properties of Prox1+ cells (referred to as SLYM or a fourth meningeal layer (Møllgård et al., 2023; Jan 2023 news).
In Supplemental Figure 3a, HRP (45 kDa) reaches the AB layer (including being taken up in vesicles by AB cells), arguing against size-restrictive barrier properties of adjacent inner arachnoid cells, in particular Prox1+ cells, as had been reported by another group (Møllgård et al., 2023). Notably, HRP passes into the subpial space from the SAS, consistent with reports by Mapunda et al., 2023, that the pial layer, though continuous and containing VE-cadherin junctions, does not form a size restrictive barrier. Notably, Smyth et al.'s EM in Figure 1C also shows arachnoid barrier and arachnoid fibroblasts fused above a single subarachnoid space. This matches recent studies by Pietilä et al., 2023, and decades of EM studies on a variety of species, supporting a single arachnoid mater comprised of fused sublayers (Sep 2023 news).
Ideally, these types of studies could be repeated by combining Dpp4-CreER (AB) and Prox1-GFP (inner arachnoid) both in EM and in 2-photon live imaging to help resolve recent controversies regarding functional meningeal anatomy and CSF flow.
Overall, I find this to be a timely, innovative and high-interest study using numerous advanced techniques that will enable future work on peripheral-CNS communication in health and disease.
References:
Yao H, Price TT, Cantelli G, Ngo B, Warner MJ, Olivere L, Ridge SM, Jablonski EM, Therrien J, Tannheimer S, McCall CM, Chenn A, Sipkins DA. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature. 2018 Aug;560(7716):55-60. Epub 2018 Jul 18 PubMed.
Møllgård K, Beinlich FR, Kusk P, Miyakoshi LM, Delle C, Plá V, Hauglund NL, Esmail T, Rasmussen MK, Gomolka RS, Mori Y, Nedergaard M. A mesothelium divides the subarachnoid space into functional compartments. Science. 2023 Jan 6;379(6627):84-88. Epub 2023 Jan 5 PubMed.
Mapunda JA, Pareja J, Vladymyrov M, Bouillet E, Hélie P, Pleskač P, Barcos S, Andrae J, Vestweber D, McDonald DM, Betsholtz C, Deutsch U, Proulx ST, Engelhardt B. VE-cadherin in arachnoid and pia mater cells serves as a suitable landmark for in vivo imaging of CNS immune surveillance and inflammation. Nat Commun. 2023 Sep 20;14(1):5837. PubMed.
Pietilä R, Del Gaudio F, He L, Vázquez-Liébanas E, Vanlandewijck M, Muhl L, Mocci G, Bjørnholm KD, Lindblad C, Fletcher-Sandersjöö A, Svensson M, Thelin EP, Liu J, van Voorden AJ, Torres M, Antila S, Xin L, Karlström H, Storm-Mathisen J, Bergersen LH, Moggio A, Hansson EM, Ulvmar MH, Nilsson P, Mäkinen T, Andaloussi Mäe M, Alitalo K, Proulx ST, Engelhardt B, McDonald DM, Lendahl U, Andrae J, Betsholtz C. Molecular anatomy of adult mouse leptomeninges. Neuron. 2023 Dec 6;111(23):3745-3764.e7. Epub 2023 Sep 29 PubMed.
Department of Pathology, RUSH Medical College
The authors provide an important cellular and functional description of the arachnoid barrier cell layer that explains how it is simultaneously capable of separating and adjoining compartments (namely, the subarachnoid space and dural interstitium), thereby accomplishing barrier functions while also permitting fluid and cellular communication between meningeal layers in mice.
They refer to arachnoid barrier defects next to veins as arachnoid cuff exits or “ACE” points, that correspond to tracer hot spots. The concept of ACE points is concordant with recent in vivo human data from individuals with Alzheimer’s disease, that suggest convergence of efflux fluid at junction points around meningeal cerebral veins as they enter dura (Mehta et al., 2023). This conception of CNS fluid flow updates knowledge on brain-body connections and physiology that has been perplexing historically. It is an important finding for interpreting and investigating disease pathogenesis in the brain, and will be interesting to study in Alzheimer’s disease.
References:
Mehta RI, Carpenter JS, Mehta RI, Haut MW, Wang P, Ranjan M, Najib U, D'Haese PF, Rezai AR. Ultrasound-mediated blood-brain barrier opening uncovers an intracerebral perivenous fluid network in persons with Alzheimer's disease. Fluids Barriers CNS. 2023 Jun 16;20(1):46. PubMed.
Rockefeller Neuroscience Institute of West Virginia University
This interesting study examined mouse and human perivenous physiology, which has been a relatively overlooked area of investigation. The authors found that dynamic changes occur in both species within the spaces around veins, and further depict immune cellular changes at the sites of dural entry by veins in mice, where there are gaps in the arachnoid barrier cell layer. Further exploration of the imaging changes in perivenous and arachnoid cuff exit (ACE) regions may elucidate neurobiology of diseases. Moreover, understanding ways in which the physiology of ACE regions can be therapeutically targeted and/or modulated in humans will be valuable.
Make a Comment
To make a comment you must login or register.